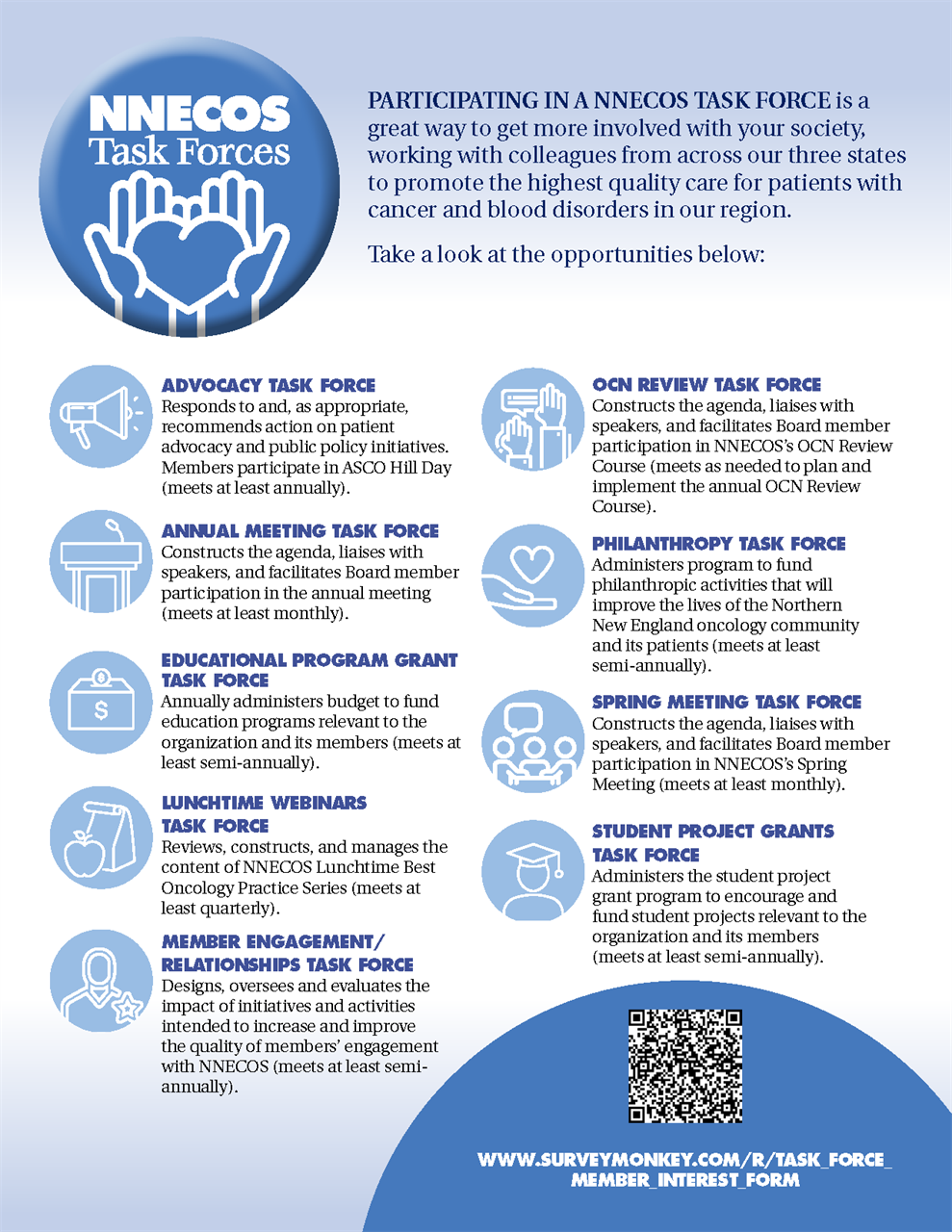
The mission of NNECOS is to promote the highest quality care for patients with cancer and blood disorders in our region through professional networking, education, and scientific research, as well as patient advocacy and public policy.
UPCOMING EDUCATIONAL OPPORTUNITIES |
|
RESEARCH, PROJECT & PROGRAM FUNDING |
NNECOS PATIENT PHILANTHROPY
OTHER NEWS & INFORMATION |
|
|
THANK YOU DIAMOND & PLATINUM CORPORATE MEMBERS

 |
|
|
|
|

|  |
|
|
|
|
|
|
|
|
|
|
|
View the complete list of corporate members.





.png)