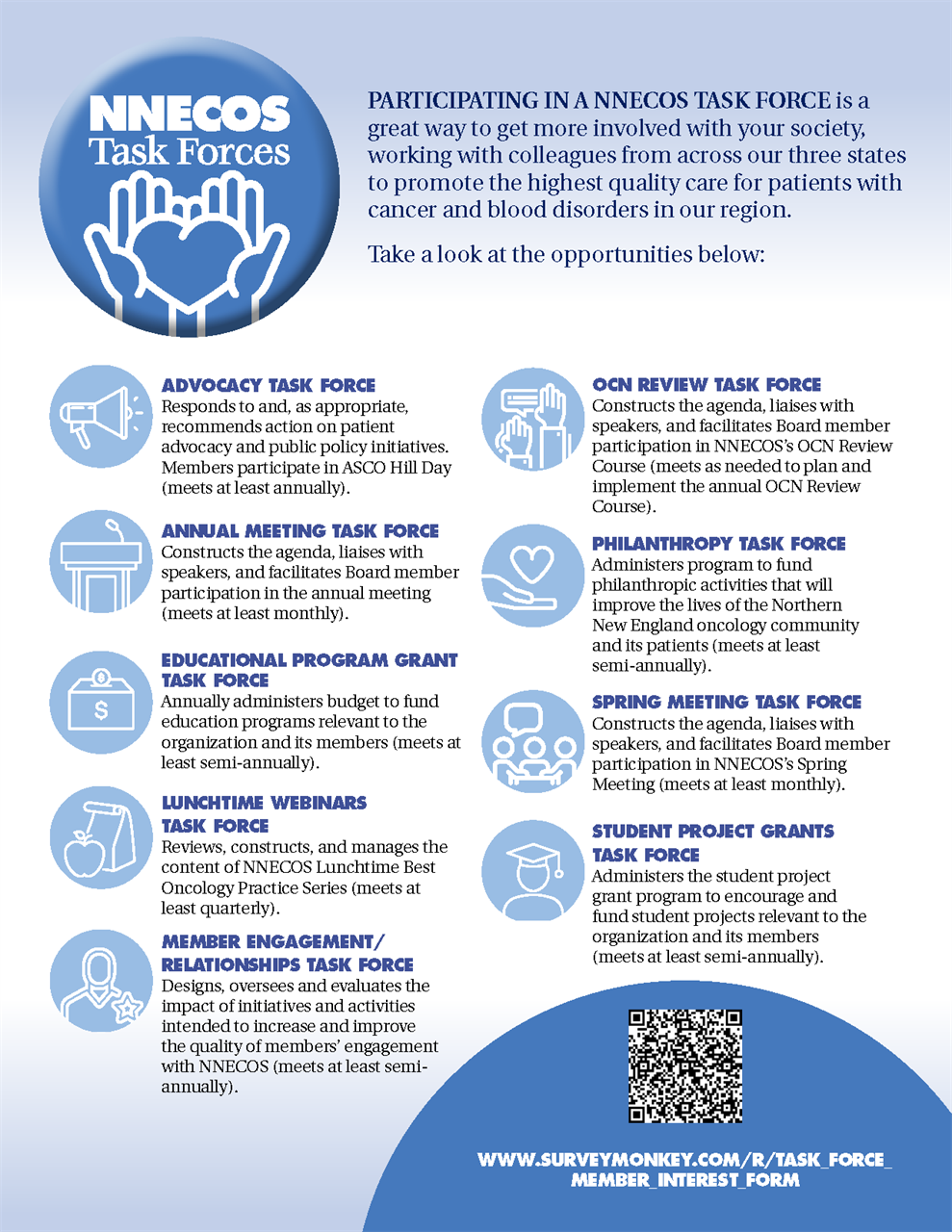
The mission of NNECOS is to promote the highest quality care for patients with cancer and blood disorders in our region through professional networking, education, and scientific research, as well as patient advocacy and public policy.
Register for the NNECOS Spring Meeting by March 17th to be entered into our St. Patrick’s Day raffle! One lucky winner will receive a complimentary one-night stay at The Lodge at Spruce Peak during the 2026 Spring Meeting. |
ALL UPCOMING EVENTS & OPPORTUNITIES |
|
|
RESEARCH, PROJECT & PROGRAM FUNDING |
NNECOS PATIENT PHILANTHROPY
OTHER NEWS & INFORMATION |
|
|
THANK YOU DIAMOND & PLATINUM CORPORATE MEMBERS

 |
|
|
|
|
|

|  |
|
|
|
|
|
|
|
|
|
|
|
View the complete list of corporate members.







.png)