Accepted Abstracts
Abstract Subgroup: Case Reports
Immune-mediated toxicity of pembrolizumab: A case of myasthenia gravis
A. Baronner, R. Zhang, K. Shirai
Dartmouth Hitchcock Medical Center, Dartmouth Geisel School of Medicine
Background: Pembrolizumab, a checkpoint inhibitor that blocks programmed cell death receptor 1 (PD-1), has been approved by the FDA for treating malignancies such as melanoma and non-small cell lung cancer. Pembrolizumab can cause immune-mediated adverse events such as pneumonitis, colitis, hepatitis, and endocrinopathies. Although rare, myasthenia gravis is an emerging and potentially fatal adverse event associated with pembrolizumab.
Methods: An 87-year-old gentleman with metastatic melanoma who had received two cycles of pembrolizumab presented with sudden-onset left facial droop, left ptosis, diplopia, and head drop. CT head and MRI were negative for acute infarct, intracranial hemorrhage, or intracranial metastases. Troponin-T and CPK elevation prompted workup with cardiac MRI, which was negative for myocarditis. Laboratory findings were also significant for transaminitis.
Results: We suspected the etiology of this presentation to be pembrolizumab-associated myasthenia gravis despite negative acetylcholine receptor antibody. Electromyography revealed neuromuscular junction defect affecting the ocular muscles, proximal muscle myopathy, and peripheral neuropathy consistent with myasthenia gravis. Treatment with pyridostigmine 30mg TID and prednisone 20mg daily was initiated. Respiratory function was monitored with negative inspiratory force measurement. Left ptosis, facial droop, and bulbar muscle weakness have subjectively improved with ongoing treatment.
Conclusions: Myasthenia gravis is a rare but emerging form of immune-mediated adverse events secondary to checkpoint inhibitors such as pembrolizumab. Troponin and CPK elevation may be related to myasthenia gravis. Close monitoring and prompt treatment are indicated given the fatal consequence of ventilatory failure secondary to respiratory muscle weakness.
A rare case of Di Guglielmo syndrome with refractory thrombocytopenia
B. Batukbhai, R. Ramkissoon, C. Locke Jr.,R. Zhang,
M. Gautier
Dartmouth Hitchcock Medical Center
Background: Giovanni Di Guglielmo first described pure erythroid leukemia (PEL), a rare and aggressive form of acute leukemia, in the early 20th century. PEL is characterized by the presence of >80% of erythroid precursors in the bone marrow, of which 30% or more are proerythroblasts.
Methods: A 44-year-old female was transferred from the referring hospital with profound anemia, thrombocytopenia that was refractory to platelet transfusion and transaminitis. Imaging was remarkable for a uterine leiomyoma, bilateral hemorrhagic ovarian cysts and ascites; she was also noted to have hepatomegaly but not splenomegaly. Bone marrow biopsy performed revealed almost 100% cellularity with >80% erythroid precursors and abnormal megakaryocytes. Staining also revealed significant fibrosis of the marrow. Cytogenetics studies could not be performed due to insufficient sample.Results: Patient was diagnosed with acute erythroid leukemia and was treated with the 7+3 chemotherapy regimen. She received IVIG and prednisone for presumed immune-related thrombocytopenia, without improvement. Screening for allo-antibodies was negative. Repeat CT abdomen showed new splenomegaly, hence the refractory thrombocytopenia was attributed to sequestration as well as decreased production. The transaminitis was thought to be from extramedullary involvement of the liver. Patient is currently scheduled for day 14 bone marrow biopsy to evaluate her response to the induction chemotherapy.
Conclusions: PEL is a rare leukemia associated with poor prognosis with standard induction chemotherapy. Patients with PEL often present with profound cytopenias and have complex cytogenetics and poor-risk molecular abnormalities. Allogeneic stem cell transplantation should be considered if remission is achieved as they have high risk for relapse.
Case series of treatment-related MDS/AML
B. Batukbhai, A. Caffrey, B. Hale, C. Hayes
Dartmouth Hitchcock Medical Center
Background: About 5-20% of patients who received chemotherapy or radiation for prior conditions develop secondary treatment-related MDS or AML(t-MDS/AML). T-MDS/AML is typically associated with abnormal cytogenetics and therefore carries a poorer prognosis than conventional AML. Historically, these conditions were classified based on the cytotoxic exposure, however with the 2008 revision of WHO classification, they are now classified based on cytogenetic changes.
Methods: We present a case series of 6 patients who developed t-MDS/AML following various cytotoxic treatment regimens. These patients were seen in the hematology clinic or inpatient ward at our institution over the last 3 years.
Results: We included 3 patients with breast cancers, 2 with lymphoma and 1 with multiple myeloma.
The average age of the patients 56.5 years (range 53-65). The time from the initial chemotherapy to the development t-MDS/AML ranged from 9 to 69months. 3 patients had received anthracycline-based treatment, 2 with bendamustine-based regimens and 1 with Cytoxan conditioning. All but one patient presented with t-AML.
2 patients were positive for MLL/11q23 rearrangement, while 2 other patients had complex karyotypes. Interestingly, the MLL/11q23 rearrangement was associated with the shortest time (9-10 months) to the development of the t-MDS/AML. 5 patients had received G-CSF with their prior chemotherapy, while 4 had prior smoking history.
Conclusions: Cytotoxic treatment such as alkylating agents, anthracyclines and topoisomerase inhibitors are associated with higher risk of t-MDS/AML, which is associated with poor prognosis. Hence, there is increasing necessity to study potential biomarkers/genetic markers to identify patients at higher risk of developing t-MDS/AML.
Comprehensive management of a patient with decreased shoulder function and a history of breast, lung, and neck cancer: a case study
A. Chongaway, A. Litterini
University of New England
Background: Cancer treatments can have late effects on the musculoskeletal, cardiopulmonary, nervous, and integumentary systems. This case report describes comprehensive physical therapy (PT) management of decreased shoulder function for a patient with a history of breast, lung, and tongue cancers treated with surgery, chemotherapy, and radiation.
Methods: The patient was a 71-year-old female referred to outpatient PT for right shoulder and neck pain with the goals to reduce pain and increase functional mobility. Local cancer treatment occurred to the right upper quadrant for multiple cancers. Outcome measures included the Upper Extremity Functional Scale (UEFS), the Numeric Pain Rating Scale (NPRS), range of motion (ROM) assessment and strength testing Interventions included manual therapy, therapeutic exercises, and aquatic therapy.
Results: The patient received nine visits of skilled PT. At discharge, she demonstrated improvement in right shoulder flexion ROM (146 to 155 degrees) and strength (4/5 to 4+/5). She reported improvements in lifting overhead, though her UEFS score improved minimally (72/80 to 74/80). On the NPRS, her pain decreased (6/10 to 1/10).
Conclusions: This case report described a comprehensive PT plan for decreased shoulder function in a patient with a history of multiple cancers. Late effects of cancer treatment can have a significant impact on a patient’s ability to complete essential activities of daily living and substantially decrease quality of life. Research has shown PT to be beneficial in mitigating these late effects, and therefore, rehabilitation should be included in the comprehensive care of cancer survivors.
Fatal delayed diagnosis of Hemaphagocytic Lymphohistiocytosis in a stroke patient
A. Donovan
Dartmouth Hitchcock Medical Center
Background: Diagnosis of Hemaphagocytic Lymphohistiocytosis (HLH) is often delayed due to its non-specific diagnostic criteria and unfamiliarity with the syndrome; however, early diagnosis is essential for treatment. Here we present a case of a delayed diagnosis despite an unexplained fever of 3 weeks in an ICU patient.
Case Presentation: A 68 year old woman presented with weakness and was found to have a stroke. Within 3 days, she developed fevers, hemoptysis, and ARDS requiring intubation. Fevers and ongoing respiratory failure persisted for 3 weeks despite extensive infectious workup (negative bacterial cultures, viral studies, and imaging) and empiric broad spectrum antibiotics for 14 days. Rheumatologic, hematologic and solid malignancies were ruled out via serologic testing and imaging, after which HLH was considered.
Pertinent findings included pancytopenia, hepatomegaly, ferritin 4122 (nl 30-400), D-Dimer 3763 (nl 0-500), triglycerides (399), CD25 1315 (nl <1000), NK function < 1.0 (nl 5.8 – 59.2), and bone marrow biopsy with hemophagocytosis (without evidence of malignancy). HScore prior to NK and CD25 levels resulted suggested a 91% likelihood of HLH; however, unfamiliarity with the diagnosis further delayed consideration of treatment. Once these levels returned and further supported HLH (100% likely per HScore), Etoposide was initiated; however, she unfortunately died due to irreversible ARDS.
Discussion: HLH should be considered early in patients with unexplained systemic inflammation and fevers, as mortality rates are high and therapy is most successful early in the disease course. Unfamiliarity with such a diagnosis should not limit consideration of treatment when no other explanation exists.
Advances in Metastatic Melanoma: The Role of Immunotherapy and Metastasectomy
V. Forbes, K. Shirai, C. Angeles
Dartmouth-Hitchcock Medical Center
Background: Immunotherapy has transformed metastatic melanoma treatment with 3-year survival rates over 50%. However, the median progression-free survival ranges 6.9-11.5 months. Many patients will progress and undergo resection, but their outcome is not well-defined.
Methods: We report two patients successfully treated with immunotherapy and metastatectomy.
Results: Patient 1 is a 48-year-old man diagnosed with metastatic melanoma to the lungs in 2013. He received Dabrafenib until imaging revealed an enlarging lung nodule and new gallbladder metastasis. He started Pembrolizumab in October 2015, completed lung SBRT, and underwent cholecystectomy in March 2016. He completed 36 cycles of Pembrolizumab with negative disease over 2 years after surgery.
Patient 2 is a 80-year-old man diagnosed with metastatic melanoma to the lung and bones in 2011. He completed 4 cycles of Ipilimumab. He had no disease evidence until September 2015 when an axillary node was identified. After 5 cycles of Pembrolizumab, he underwent node dissection. Re-imaging showed new metastatic lesions. Ipilimumab was restarted in June 2016. After 4 cycles, imaging revealed decreased disease burden. In December 2016, increased axillary metastasis and a new small bowel lesion were detected. Ipilimumab was re-tried for 4 cycles. A March 2017 scan demonstrated axillary node resolution and an increasing intestinal lesion. He underwent bowel metastatectomy with negative disease over 1 year after resection.
Conclusions:These patients illustrate the importance of a multidisciplinary approach and the promise of immunotherapy and surgery in the setting of metastatic melanoma.
In select patients with advanced melanoma treated with immunotherapy, metastasectomy can be associated with good outcomes.
Refractory hemolytic anemia: a case of paroxysmal cold hemoglobinuria
Allison Gathany 1, Adriane Budavari 2, Jeremy Larsen 3
1Dartmouth-Hitchcock Medical Center, 2Department of Internal Medicine, Mayo Clinic, 3Department of Hematology/Oncology, Mayo Clinic
Background: Paroxysmal cold hemoglobinuria (PCH) is a variant of cold autoimmune hemolytic anemia in which IgG antibodies sensitize red blood cells at cold temperatures causing complement fixation and intravascular hemolysis on rewarming. Diagnostic gold standard is the Donath-Landsteiner test for biphasic hemolysin IgG autoantibodies. PCH is most common in children, median age at diagnosis is 5 years. Onset is often triggered by viral illness. We report a case of PCH in a 27-year-old woman presenting in hemolytic crisis.
Methods: Case report
Results: Our 27-year-old patient with history of anemia presented to an outside hospital with severe symptomatic anemia (hemoglobin 4 g/dL) and evidence of hemolysis including undetectable haptoglobin. Work-up included negative Coombs, G6PD, cryoglobulins, RBC band3 screen for hereditary spherocytosis, and infectious workup including parvovirus. CT abdomen/pelvis showed splenomegaly with multiple splenic infarcts. She underwent splenectomy due to refractory transfusion-dependent hemolysis; pathology was benign. On transfer of care she was symptomatically anemic with hemoglobin 6.8 g/dL. Empiric steroid therapy (methylprednisolone 1g daily for three days) stabilized her hemoglobin around 8 g/dL. Testing was positive for Donath-Landsteiner antibody, negative for cold agglutinin and PNH. She was discharged on prednisone 40mg daily which was tapered. At follow-up seven months later, after three months off prednisone, she had normal hemoglobin with no hemolysis. She was counseled to avoid cold exposure.
Conclusions: This case of laboratory-proven PCH in a 27-year-old woman with hemolytic crisis, and delayed diagnosis of this rare condition resulted in unnecessary splenectomy. She had excellent response to steroids and recovered well.
Insulin-related amyloidosis in a patient with type 2 diabetes and colon cancer.
A. Harb, A. Merrill Garrett
Eastern Maine Medical Center Cancer Care
Background: Localized insulin-derived amyloidosis (LIDA) is an extremely rare complication of insulin injections. It could result in poor glycemic control secondary to decreased insulin absorption. LIDA this is not associated with systemic amyloidosis and does not warrant systemic therapy.
Methods: We report the case of a 71-year-old the man with severe significant multiple medical issues who presented in early 2018 with increased abdominal pain and thickening of the abdominal wall. CAT scan of the abdomen and pelvis showed a left lateral wall soft tissue mass as well as the transverse colon lesion. The biopsy of the colon mass was in favor of a colon adenocarcinoma.
Further tests were performed on the skin lesion, including Congo red, and liquid chromatography tandem mass spectrometry (LC MC/MS). The latter test detected the presence of a peptide profile consistent with Alns (insulin)-type amyloid deposition.
Results: None
Conclusions: The patient is undergoing treatment for his colon cancer.
Very few cases have been reported in the literature about insulin-related amyloidosis. It is an entity that does not warrant systemic therapy. Some reports have recommended surgical resection of the amyloidoma in case of poor glycemic control.
Conservative medical management of pneumatosis intestinalis in association with graft-versus-host disease after allogeneic stem cell transplant
D. Huang 1, M. Wesley 2, J. Hill Jr 3
1Geisel School of Medicine at Dartmouth, 2Department of Medicine, Dartmouth-Hitchcock Medical Center, 3Blood and Marrow Transplant Program
Background: Pneumatosis intestinalis (PI) is a rare radiographic finding of air in the bowel wall, with clinical severity ranging from self-limited to acutely life-threatening. While management has varied from supportive medical intervention to, more commonly, surgical exploration, PI-related mortality has been increasingly associated with surgical complications, rather than the progression of PI, itself. We present a case of PI related to post-transplant graft-versus-host disease (GVHD) at our transplant center to illustrate its successful non-surgical management.
Methods: Case Report
Results: A 53-year-old man was admitted for progressive, non-bloody diarrhea and crampy abdominal pain four months after allogeneic stem cell transplant (ASCT) for myelodysplastic syndrome.
Gastrointestinal GVHD was confirmed with biopsy, prompting initiation of methylprednisolone and tacrolimus. On hospital day twelve, his abdominal pain and distention worsened, with laboratory studies showing leukocytosis (12,200/uL) and serum lactate elevation (2.2mmol/L). A computed tomography of abdomen/pelvis showed extensive colonic pneumatosis and mesenteric venous gas, concerning for diffuse bowel ischemia. General Surgery was consulted, and diagnostic laparoscopy with open bowel resection was considered. In the absence of definitive free air and presence of clear surgical risks, including steroids and other immunosuppression, a trial of medical management was chosen. The patient was treated conservatively with bowel rest, parenteral antibiotics, and total parenteral nutrition, with close clinical and radiographic follow-up. He demonstrated steady improvement and was discharged home on oral medications, without surgery.
Conclusions: This case demonstrates the feasibility of medical management for PI associated with GVHD after ASCT to minimize morbidity and length of hospital stay.
Cutaneous presentation of a T Lymphoblastic Lymphoma
X. Jordan Bruno
University of Vermont Medical Center
Background: T lymphoblastic lymphoma is an uncommon entity accounting for only 1 % of lymphomas in the adult population. The typical presentation of this condition consists in shortness of breath secondary to mediastinal, pleural and lung involvement with large mediastinal masses being its hallmark. This in an aggressive disease being the mirror counterpart of T Acute Lymphoblastic Leukemia. Even though involvement of the skin and central nervous system have been described is it very unusual that this would be the clinical debut of this disease
Methods: Our patient presented with a history of two weeks of appearance of painless rapidly growing skin nodules in his scalp area. He initially consulted to his dermatologist who performed a punch biopsy of these lesions. Biopsy showed infiltration by very immature lymphocytes which co expressed CD4 and CD8 and with a very high percentage of Ki67 (close to 100 %)
Results: Shortly after his initial diagnosis his clinical scenario deteriorated and he developed shortness of breath with exertion, PET /CT scan confirmed extensive mediastinal mass , as well as malignant pleural effusion which was tapped .Flow cytometer analysis confirmed same phenotype in these lymphocytes as cells identified in the initial skin biopsy
Patient was started on an age adjusted Hyper C VAD regimen
Conclusions: T Lymphoblastic Lymphoma is a very unique entity in its clinical presentation. It still represents a therapeutic challenge especially in the elderly population.
Cutaneous T-cell lymphoma lesions with superimposed Norwegian scabies
M. Keating, L. Giscombe, N. Reddy, R. Rathore
Roger Williams Medical Center/Boston University
Background: 65-year-old male with past medical history of cutaneous T-cell lymphoma diagnosed five years ago, hypertension, congestive heart failure, and diabetes mellitus, presented for abnormal labs and weakness. Patient was previously treated with nbUVB but stopped treatments two years ago due to noncompliance. Exam revealed poor hygiene and diffuse erythrodermic skin involvement with heavy crusting as thick as three cm on his face, abdomen, back, and extremities. There was palpable inguinal lymphadenopathy on exam. Our approach was to re-stage him and to rule out progression from cutaneous T-cell lymphoma to Sezary syndrome. CT scans, T cell panel, and peripheral flow cytometry were checked, and dermatology was consulted for skin lesion biopsy. He was determined to have stage 2A/2B disease and the workup did not support progression to Sezary syndrome. Ultimately, he was started on methotrexate due to social issues precluding more involved regimens. However, given the remarkably thick crusted lesions there was early concern for Norwegian scabies during his hospitalization. The patient was treated with empiric permethrin and ivermectin and kept on isolation. The first skin biopsy was equivocal, and the second skin biopsy was negative after empiric treatment had been started. He was continued on empiric treatment upon discharge as a precaution. We found this case to be particularly instructive in the recognition of rare but highly transmissible cutaneous infections in an immunocompromised host. Early identification, initiation of empiric treatment, proper isolation, and notification of involved health care workers were key to minimizing the risk to the patient and staff.
Methods: N/A
Results: N/A
Conclusions: N/A
Mixed Epitheliod Trophoblastic Tumor and Choriocarcinoma, a Diagnostic Conundrum
K. Victoria, H. Rehman
University of Vermont Medical Center
Background: There are three subtypes of gestational trophoblastic disease (GTD), choriocarinoma being the most common, placental site trophoblastic disease (PSTT) and epitheliod trophoblastic tumor (ETT). Gestational choriocarcinoma, is very chemosensitive and potentially curable even at a late stage, whereas non-gestational, ETT/PSTT are not. A mixed tumor composed of choriocarcinoma and ETT is very rare. We present a case of a 66-year-old woman diagnosed with a mixed tumor.
Methods: A 66-year-old woman presents to her gynecologist with several months of post-menopausal bleeding. CT scan of the abdomen showed a 10-cm pelvic mass, consistent with high grade carcinoma. Her B-HCG was 8914. Pt underwent TAH-BSO. Additional staging with MRI head revealed multiple subacute cerebral infarcts. Patient was admitted to the hospital and started on heparin drip. She proceeded to develop hypoxic respiratory. CT scan of the chest demonstrated extensive pulmonary metastases. She received one cycle of cisplatin 20 mg/m2 and etoposide 100 mg/m2 (daily x 5 days). The patient unfortunately developed severe mucositis, hemodynamically significant vaginal bleeding and additional sites of thrombosis.
Results: B-HCG came down to 3500. A repeat CT demonstrated no change in the size of her lung nodules. The patient developed multiple intracranial hemorrhages. Foundation one and paired allele microsatellite testing are still pending
Conclusions: Previous case reports have noted that both ETT and non-gestational choriocarinoma are not chemosensative. Presently, we do not know if our patient’s tumor is gestational. GTD would be comprised of part paternal DNA. Two explanations for our patient’s current lack of response to chemotherapy are that the highest burden of metastatic disease is ETT or non-gestational. The ratio of choriocarinoma to ETT may be an explanation to why case reports' of GTD mixed tumors have such a varied response to chemotherapy.
Acute Oxaliplatin Immune-Induced Syndrome: A Case Report
S. Waldstein 1, C. Gilkey 1, M. Barry 2
1University of Vermont Medical Center, 2University of Vermont Medical Center Division of Hematology/Oncology
Background: Oxaliplatin is a common third-generation platinum analog used to treat gastrointestinal malignancies, particularly colorectal cancer (CRC). In cases of CRC, the drug combination FOLFOX has been shown to increase survival and reduce recurrences. The most common side effects of oxaliplatin include peripheral sensory neurotoxicity, nausea, vomiting, hematologic toxicity, and diarrhea. However, there are also rare and severe side effects, including oxaliplatin immune-induced syndrome (OIIS).
Methods: 81 year old woman with metastatic colon cancer treated with FOLFOX plus bevacizumab who was admitted to the hospital directly following administration of cycle 2 of oxaliplatin due to back pain and hematuria. She was additionally febrile to 101.4 and mildly hypoxic.
Results: Labs showed a marked leukocytosis, as well as thrombocytopenia and evidence of hemolysis with elevated LDH and total bilirubin. Direct antiglobulin test and M antigens were positive. Cell bound platelet antibody screen was negative. Urinalysis was positive for blood and protein. She was initiated on prednisone 40mg and IV fluids. Platelet count stabilized, leukocytosis decreased, and hypoxia resolved. Hemoglobin stabilized and markers of hemolysis improved. She was discharged on a prednisone taper with continued improvement.
Conclusions: This case report highlights a rare and serious side effect of oxaliplatin. Documentation of these cases is essential for early recognition and intervention.
Abstract Subgroup: Genetic testing, molecular findings, etc
Community oncology clinicians’ knowledge, beliefs, and attitudes regarding genomic tumor testing
E. Anderson 1, K. Murray 1, H. Mandeville 1, C. Gutheil 1, L. Waterston 1, L. Lucas 1, C. Duarte 1, C. Thomas 2, S. Miesfeldt 3, P. Helbig 4, A. Antov 4, J. Rueter 4, P. Han 1
1Center for Outcomes Research and Evaluation, Maine Medical Center Research Institute, 2New England Cancer Specialists, 3Cancer Risk and Prevention Program, Maine Medical Center, 4Jackson Laboratory
Background: Genomic tumor testing (GTT) is a new technology that promises to make cancer treatment more precise, but little is known about clinicians’ knowledge, beliefs, and attitudes regarding GTT, particularly in community oncology settings.
Methods:
64 Maine-based oncology clinicians completed a survey, as part of their participation in the Jackson Laboratory Maine Cancer Genomics Initiative (MCGI), designed to assess their past and anticipated future testing practices, and their knowledge, beliefs, and attitudes regarding GTT.
Results: Eighty-one clinicians were contacted and invited to join this study, and 64 (52% female) agreed to participate (79% response rate). Fifty-eight percent of participants reported practicing in rural or small town settings. Seventy percent reported they ordered GTT in the past 12 months and 88% reported they would order GTT in the next 12 months. Forty-eight percent of participants reported a high degree of confidence in their ability make appropriate treatment decisions based on GTT. When asked to identify potential problems with GTT, clinicians were most concerned with: lack of insurance coverage, low likelihood of identifying actionable variants, and obtaining adequate tissue samples. Eighty-one percent believed that GTT is beneficial and only 2% believed that GTT is harmful.
Conclusions: Oncology clinicians practicing in community settings report relatively high current use of GTT and higher anticipated use in the future. As GTT becomes more widespread, the knowledge, attitudes, and educational needs of community-based oncology clinicians will need to be understood and addressed.
Genetic Testing Process Improvement
K. Hall, M. Clements
Lahey Oncology/Hematology at Parkland Medical Center
Background: Genetic testing has become an important component to determining treatment options. The process involves multiple parties leading to inefficiencies and potentially a delay in results. This "genetic maze" was identified as an opportunity to streamline the process, allowing for a more consistent workflow with genetic testing.
Objectives
Global Aim: Standardize genetic testing process to create formalized and consistent method once genetic testing ordered. Specific Aim: Decrease turnaround time for test results to avoid delay in care.
Methods: Created a Genetic Testing tracking log allowing for streamline communication on all genetic tests ordered to avoid delay in process when provider/MD nurse unavailable.
Identified point person with genetic testing company to help expedite process and have regular communication regarding next steps and necessary documentation needed to begin testing.
Results: Collected data on total number of days for test results (date test ordered to when test results reported to provider) which showed the turnaround time for test results was quicker by 4 days.
Current State
All genetic tests ordered are logged into Genetic Testing spreadsheet for tracking purposes.
Nurse Navigator designated as "assigned delegate" for online portal to expedite access to timely results.
Communication pathway created for genetic test results prior to breast surgery to ensure consistent communication process with interdisciplinary team.
Conclusions: Standardization of genetic testing has proven to decrease turnaround time for test results.
Limitations: PI focused on BRCA testing only during 8-month time period. We have since expanded to track other driver mutations as well as genomic testing.
MET amplification in non small cell lung cancer: experience of a single institution in Maine.
A. Harb, A. Curtis
Eastern Maine Medical Center Cancer Care
Background: MET (Mesenchymal Epidermal Transition) amplification has been recently recognized as a targetable mutation in non-small cell lung cancer (NSCLC). According to the National Comprehensive Cancer Network (NCCN) guidelines, patients with MET positive NSCLC can be treated with crizotinib.
Methods: We examined all lung adenocarcinomas diagnosed at Eastern Maine Medical Center (EMMC) between 2015 and 2018. Patients with MET positive NSCLC were identified. Other lung driver mutations and PD-L1 status were also studied in these patients.
Results: Among 382 patients tested, 23 had MET amplification (6%), 20 of whom were treated at EMMC. Only one patient had co-EGFR mutation (exon 19 deletion). Non-had other coexisting mutation. 11/20 (55%) had a high PD-L1 expression (>50%). 95% were current or former smokers. 13 patients were diagnosed with stage IV, 4 patient with a stage III and 3 patients with stage I disease. Among the 13 patients with stage IV disease, 5 received immunotherapy, 4 chemotherapy, 1 received erlotinib, 1 did not receive any treatment, and only 2 received crizotinib.
Conclusions: MET amplification was present in about (6%) of patients with lung adenocarcinomas. It seems that this subgroup of patients has an increased incidence of high PD-L1 expression. Even though crizotinib is approved for patients with stage IV MET positive NSCLC, it is not likely to be the first line of therapy in most cases (15%).
Next generation sequencing in detecting KRAS and p53 mutations in non-small cell lung cancer patients from rural Maine.
A. Harb 1, L. Skacel 2, M. Babcock 2, K. T. Brawn 2, C. L. Liou 2, M. Skacel 2
1Eastern Maine Medical Center Cancer Care, 2Dahl-Chase Diagnostic Services,
Background: KRAS and p53 are two commonly identified mutations in cancer. Next Generation Sequencing (NGS) has recently become a widespread available technology, permitting DNA profiling in cancer on the large-scale. It is becoming a standard of care in modern oncology allowing more “personalized” medical care.
Methods: 100 cases of non-small cell lung cancer (NSCLC) were tested by NSG between January 2017 and January 2018 at Dahl-Chase Diagnostic Services in Bangor. Specimens were obtained from 40 men and 60 women aged 45-72 years. The median age was 62 years. A 50 gene analysis was conducted using cancer “Hotspot” Panel V2. Only KRAS and p53 mutation results were selected for the study.
Results: KRAS mutations were identified and 34 cases (34%). The most commonly detecting mutation was in codon 12 (G12V and G12C) (74%). The second most common was in codon 13 (15%). 9% were seen in codon 61. 97% were localized in the “Hotspot” regions.
P53 mutations were detected in 52 cases (52%). There were a wide variety of mutations identified with the most common being point mutation pY163C and pR248Q (5 in 4% respectively). The “Hotspot” mutations counted only for 14% of the p53 mutations.
3 cases had co-expression of KRAS and p53 mutations (3 different p53 abnormalities).
Conclusions: Our analysis of the KRAS and p53 mutations in NSCLC in Eastern/Northern Maine by NGS showed a distribution of mutations similar to what was previously reported in the literature.
NSCLC: Acquisition Of Potentially Novel Resistance Mutations
W. Jehangir, F. B. Khan, D. J. Seward
University of Vermont Medical Center
Background: CA 71-year-old woman with no smoking history underwent imaging for right shoulder pain and was found to have a 2 cm speculated right upper lobe mass.
Methods: Biopsy revealed poorly-differentiated non-small cell lung (NSCL) adenocarcinoma. PET imaging showed retroperitoneal lymphadenopathy and bony metastasis which was also confirmed by biopsy. She was found to have an EGFR exon 19 deletion on tumor genomic testing using a solid tumor gene panel developed at University of Vermont. She was enrolled on a phase III clinical trial of erlotinib versus osimertinib in September 2015. After 14 months, imaging showed progressive disease. A repeat biopsy and genomic analysis was performed showing a novel T790M mutation. In December 2016, she was un-blinded per study protocol and crossed over to osimertinib. After 8 months, restaging imaging showed progressive disease in her lungs, along with a right pleural effusion and pulmonary nodule. Repeat genomic testing and PDL-1 staining showed the previous EGFR Exon 19 deletion, the T790M resistance mutation as well as new CDKN2a and CTNNB1 mutations and 0-1% PDL-1 expression.
Results: Tyrosine kinase inhibitors were stopped and imaging after 4 cycles of carboplatin and pemetrexed showed progression of disease. Chemotherapy was discontinued and nivolumab was started. Further progression was seen after 2 months of therapy, and she was switched to docetaxel. She ultimately died after 5 cycles.
Conclusions: This case shows a novel resistance mechanism to this third generation tyrosine kinase inhibitor and illustrates the paradigm of controlling this cancer through cycles of drug sensitivity and resistance mutation acquisition.
The effect of extended genomic panel testing on treatment decisions for patients with metastatic breast, lung or colon cancer.
C. Lyall, C. A. Thomas
New England Cancer Specialists
Background: Extended panel molecular testing (EPMT) is utilized increasingly in patients with advanced cancer for whom standard of care treatment options are no longer available. Recent research suggests that for a variety of reasons EPMT results affect treatment decisions only 5-10% of the time. Our study aimed to assess how many patients with breast, lung, or colon cancer in a community oncology practice undergo EPMT and the effect of test results on treatment decisions.
Methods: From 1/1/2016-6/15/2018, we identified patients with stage IV breast, lung or colon cancer for whom EPMT results were available. Test results and treatment decisions for each patient were recorded.
Results: A total of 157 patients with breast cancer, 125 patients with colon cancer, and 327 patients with lung cancer were identified. 14% (n=22) of breast cancer patients, 20% (n=65) of lung cancer patients, and 13% (n=16) of colon cancer patients had retrievable EPMT results. 66% (n=43) of lung cancer patients received treatment based on EPMT results, whereas none of the colon or breast cancer patients were treated based on their EPMT results. Data will be updated to include reasons for non-treatment.
Conclusions: EPMT is utilized more commonly in patients with stage IV lung cancer as opposed to patients with stage IV colon or breast cancer. Available treatment options, physician, and patient preference may be reasons for this disparity. We are currently further determining which factors are linked to treatment decisions.
Physician-patient communication about genomic tumor testing: perceptions of oncology providers
H. Mandeville 1, E. Anderson 1, K. Murray 1, C. Gutheil 1, L. Waterston 1, L. Lucas 1, C. Duarte 1, C. Thomas 2, S. Miesfeldt 3, P. Helbig 4, A. Antov 4, J. Rueter 4, E. Edelman 4, K. Reed 4, P. Han 1
1Center for Outcomes Research and Evaluation, Maine Medical Center Research Institute, 2New England Cancer Specialists, 3Cancer Risk and Prevention Program, Maine Medical Center, 4Jackson Laboratory
Background: Genomic tumor testing (GTT) is a potentially valuable new technology that can make cancer treatment more precise, but there are substantial uncertainties about its clinical value and appropriate use. Oncology physicians need to counsel cancer patients about both the value and uncertainties of GTT, but optimal communication strategies, including goals and content elements of physician-patient discussions, remain to be determined.
Methods: 76 oncology providers (physicians, nurses, practice administrators, genetic counselors) attended an annual forum of The Jackson Laboratory Maine Cancer Genomics Initiative (MCGI) in April 2018, and completed a survey including multiple-choice and open-ended questions to ascertain their views about the key goals and content elements of physician-patient discussions of GTT. Survey responses were analyzed using descriptive quantitative and qualitative methods, including software-assisted coding.
Results: Providers prioritized the communication goals of promoting informed and shared decision making and managing patient expectations. Providers also identified a variety of broad content elements as essential for physician-patient discussions about GTT: nature of GTT, uncertainties about its value for treatment decisions, and patient expectations. The most commonly identified specific content elements included logistics of testing; therapeutic implications; potential outcomes of GTT including discovery of germline variants and potential harms; uncertainty about therapeutic options; and uncertainty arising from incomplete evidence.
Conclusions: Cancer care providers identified several communication goals and ideal content elements for physician-patient discussions about GTT. Study findings will inform future efforts to understand optimal communication strategies and to design patient education and physician training programs to promote shared decision making in GTT.
Assessment of digital PCR for single day HER2 assessment
Parth Shah 1, J. Sands 2, S. Murarka 3, B. Mehta 3
1Dartmouth Hitchcock Medical Center, 2Dana Farber Cancer Institute, 3Supratech Micropath Laboratories and Research Institute
Background: 15%-25% of breast cancer neoplasms exhibit Human epidermal growth factor receptor-2(HER2) amplification as a driver .Current testing methods include Immunohistochemistry (IHC) and Fluorescence in situ Hybridization (FISH). Digital PCR (dPCR) has been explored in determining HER2 status in cases of equivocal IHC in archived samples. In this study, we assess the clinical utility of rapid chip based digital PCR to evaluate HER2 amplification status from Formalin Fixed Paraffin Embedded (FFPE) tissue with RNaseP as a control target.
Methods: 84 tissue samples were analyzed by IHC and dPCR in parallel in a double blinded manner. IHC equivocal samples were reflexed to FISH and compared to the results obtained from dPCR. Results were analyzed on the Thermofisher Cloud platform.
Results: All 9 IHC positive and 35 negative samples had similar results on dPCR using an amplification ratio threshold for a positive result of 1.8. Of 40 IHC equivocal samples, 10 were positive, 27 negative, and three equivocal by dPCR. There was 100% concordance between dPCR and FISH results. Eight samples that were unanalyzable by FISH were satisfactorily processed on dPCR. The turnaround time(TAT) was 2 and 3 days for IHC and FISH respectively with that of dPCR being 24 hours.
Conclusions: Our results demonstrate that chip based dPCR was superior to FISH for HER2 detection. Superior TAT's and objective results were obtained even with low sample input. Moreover, dPCR does not require standards for calibration enabling deployment in low resource settings.
Abstract Subgroup: Immunology, Checkpoint inhibitors
Perplexing case of immune checkpoint inhibitor-related side effects
Bhavina Batukbhai, P. Shah, K. Shirai
Dartmouth Hitchcock Medical Center
Background: In the recent years, the introduction of immune checkpoint inhibitors (ICI) has brought a paradigm shift in the treatment of metastatic melanoma. It has been noted to provide a durable response in a small percentage of patients. Despite of its clinical benefits, ICI has been noted to have various immune-related side effects.
Methods: A 79-year-old man with history of metastatic melanoma status post right upper lobe lobectomy, presented with right arm weakness and left eye ptosis 2 weeks after first dose of Nivolumab. Labs were remarkable for elevated cardiac enzymes and transaminitis. CT head was unremarkable. Cardiac MRI showed delayed enhancement in the inferolateral wall concerning for myocarditis. Neurological evaluation was consistent with myasthenia gravis (MG).
Results: Patient was diagnosed with ICI-related myocarditis, hepatitis as well as MG. He was negative for anti-acetylcholine receptor antibodies but was positive for the anti-striated muscle(anti-SM) antibodies. He was initially treated with IV immunoglobulin and was then transitioned to steroids and plasma exchange (PLEX) which was increased to daily due to worsening neurological symptoms. He was intubated for impending respiratory failure from the myasthenia and subsequently underwent tracheostomy. His course was complicated by frontal stroke, GI bleed and autonomic instability. The anti-SM antibody titer decreased with the treatment, however he continued to have neurological symptoms and Mycophenolate mofetil was added.
Conclusions: While ICI has opened up new avenues in cancer treatment, it can cause severe complications in a small group of patients. Hence, more research is needed to identify predictive biomarkers to avoid life-threatening treatment-related toxicities.
A Pilot Trial Examining Myeloid-Derived Suppressor Cells and Immune Checkpoint Regulators' Expression in Allogeneic Stem Cell Transplant Recipients Using Myeloablative Busulfan and Fludarabine
R. Mabaera 1, K. Wilcox 1, L. Root 1, D. McKenna 1, R. Noelle 2, J. M. Hill 1, C. Hayes 1, C. Lowrey 1, Z. Szczepiorkowski 1, K. Meehan1
1DHMC - Norris Cotton Cancer Center Section of Hemat, 2DHMC - Norris Cotton Cancer Center Microbiology and Immunology
Background: Chronic graft-versus-host disease (cGVHD) remains a major barrier to improving long-term outcomes following allogeneic hematopoietic stem cell transplantation (AHSCT). Novel targeted therapeutic and/or prophylactic agents for cGVHD that do not negatively affect graft-versus-tumor or increase the risk of infectious complications are needed.
Methods: To identify potential novel targets for cGVHD management, we performed extensive immunophenotyping and functional comparison of early immune cell subsets between patients with and those without GVHD in a prospective cohort undergoing AHSCT using a standardized regimen at our center. We measured the frequency and phenotype of regulatory T cells, myeloid-derived suppressors cell subsets, and expression of immune checkpoint regulators as well as interactions among these factors during early immune recovery (engraftment until day 100).
Results: To date, 15 patients were evaluated, seven (47%) of whom developed GVHD. While all patients develop an early (at engraftment and day 30) transient increase in MDSC frequency following transplant, there was tendency towards reduced monocytic MDSC arginase expression in day 30 samples from patients who later develop GVHD, suggesting reduced function in the monocytic MDSC compartment. Further, monocytic MDSC suppressive activity was impaired in day 30 samples from patients who later develop cGVHD compared to those who do not.
Conclusions: These data suggest that monocytic MDSC function in the early post-transplant period may play a role in long term GVHD suppression and lay the foundation for more detailed studies evaluating agents that may modulate this activity.
Atypical AIDP in the setting of pembrolizumab treatment
S. Muralikrishnan, L. Ronan, S. Coker, P. Rauschkolb, K. Shirai
Dartmouth Hitchcock Medical Center, Norris Cotton Cancer Center
Background: Acute inflammatory demyelinating polyneuropathy (AIDP) is a rare (<1%) but potentially fatal complication of immunotherapy. With the expanding role of immunotherapy in treating different cancers, more cases of AIDP are likely to occur. Prompt recognition and treatment are vital in the management of AIDP with treatment typically involving a single course of intravenous immunoglobulin (IVIG) or plasma exchange (PE) followed by a few weeks of recovery. The pathogenesis of AIDP usually involves the presence of autoimmune antibodies that attack peripheral nerves, which were reported in prior case reports. Fortunately, relapses are rare, occurring in about 10% of cases.
Methods: We present a case of a 65 year old female with malignant melanoma who developed pembrolizumab induced AIDP after only two doses.
Results: The patient’s clinical course was atypical for a few reasons. Diagnostically, there were no autoimmune antibodies detected, which have been reported in other cases of immunotherapy induced AIDP. Furthermore, she had two relapses requiring treatment with multiple rounds IVIG and PE along with high doses of intravenous steroids followed by chronic oral steroids. Although she remains slow to recover, the prompt recognition of her symptoms and quick initiation of treatment helped prevent further life-threatening complications.
Conclusions: Our case report highlights an atypical presentation of AIDP in the setting of pembrolizumab use. We not only hope to make other clinicians aware of this potentially fatal complication of immunotherapy but to also show that early recognition and treatment of AIDP along with close follow up can be lifesaving.
 Cancer patients receiving immunotherapy who present to the Emergency Room; A Treatment Paradigm Shift.
Cancer patients receiving immunotherapy who present to the Emergency Room; A Treatment Paradigm Shift.
L. Pistorino, J. Dallacosta, P. Skeffington, H. McCarthy, M. Aguiar
Southcoast Centers for Cancer Care
Background: Targeted immune therapies are being approved rapidly for treatment of many cancers. These have immune mediated side effects. The treatment differs from the usual treatments associated with side effects of chemotherapy. The presenting diagnosis may be difficult for ER staff because of the similarities of the symptoms.
Methods: Retrospective study of patients receiving immunotherapy. 114 patients over a period from January 1, 2016 through Sept 30, 2017 were evaluated. Crystal reporting was used to find patients who presented to the ED and/or had a hospital admission within 30 days of infusion. Analysis included presenting symptoms, diagnosis, and treatment.
Results: Of the 114 patients, there were possibly 23 visits to ER/Hospital that could have been for immune related side effects. We evaluated the data, on diagnosis and treatment given to determine if the patient was treated appropriately.
Conclusions: We found a substantial number of patients treated for immunotherapy side effects by the emergency department with treatment modalities that were less than ideal.
Notes: Implications: The results of this retrospective study can shed some light on educational needs. Our next steps include education to ED providers and a companion immunotherapy side effect card for patients.
Abstract Subgroup: Supportive care issues – screening, care models, costs of care, rural care, behavior modifcations etc
Exercise Interventions for Breast Cancer (BC) Patients in Rural Areas: The Northern New England (NNE) Experience
B. Batukbhai 1, M. Stannard 1, A. Donovan 1, K. Lyons 1, C. Donnelly 1, M. Chamberlin 1, K. Dittus 2, K. Stoedefalke 3, J. Ockene 4
1Dartmouth Hitchcock Medical Center, 2UVM, 3Colby-Sawyer College, 4University of Massachusetts Medical School
Background: Women in rural areas are less likely to engage in physical activity (PA) compared with their urban counterparts. It can be even more challenging for BC patients due to the sequelae of their cancer and treatment
Goal setting has been an integral part of cardiac rehabilitation and behavior change techniques. It has shown to increase participation and motivation. We performed a combined analysis of collaborative interventions in NNE to identify if effective goal setting can overcome the barriers to PA among BC survivors.
Methods: 5 IRB-approved interventions promoting PA among BC patients across 3 states were stratified according to the stages of treatment, the types of intervention and whether goal setting was used. Pooled effects were calculated using Comprehensive Meta-Analysis software and R package metafor. Subgroup analyses were conducted based on the goal-setting criteria.
Results: 5 studies examining 4 exercise interventions were included. One study included patients on chemotherapy, while the other 4 included patients who were post-chemotherapy. Goal-setting was used in all the studies except for one study which used a supervised program with self-reporting.
The pooled change in PA was 56.6% (95% CI=24.2-89.1%). Pooled PA for the goal-setting subgroup was nearly twice as high as the without-goal-setting subgroup (74.3% and 39.5%, respectively).
Conclusions: Cancer centers with large catchment areas need to design creative approaches to encourage PA among the BC survivors. With the lack of motivation as the most common barrier, goal-directed programs can be helpful by creating attainable targets as well as providing emotional and psychological support.
Providing Food Assistance to Early Stage Breast Cancer Patients With Food Insecurity Significantly Improves Their Adherence to Adjuvant Therapy.
S. Caramando, E. Longnecker, T. F. Weisberg, C. A. Thomas
New England Cancer Specialists (NECS)
Background: Nationally, Maine has the third highest rate of food insecurity (FI) and may be an important factor in successfully completing adjuvant therapy for cancer patients. NECS has partnered with a Food Bank to help FI patients access food assistance. We hypothesized that FI might adversely impact stage 0-II breast cancer patients ability to complete their adjuvant treatment and that such patients might be able to complete their treatment after receiving food aid.
Methods: From 1/1/17-6/30/18, we assessed the number of patients with early stage (0-II) breast cancer undergoing adjuvant therapy (hormone and/or chemotherapy) who disclosed food insecurity and how many FI patients received food assistance (food packages) in the office. Food secure (FS) case controls matched for disease, stage, gender, and age were identified. The number of months on treatment was determined for all patients.
Results: The average number of treatment months on adjuvant therapy for FI patients (7 months) was 3.5 months shorter than for FS patients (Figure 1, p < 0.05). Receiving food assistance increased the average duration of adjuvant therapy for FI patients to 8 months, not significantly different from FS controls. (Figure 2, p > 0.05).
Conclusions: FI patients with early stage breast cancer experience a statistically significantly shorter duration of adjuvant therapy compared to food secure patients; however, the duration of treatment for FI patients can be increased significantly by providing food assistance to these patients. We conclude that receiving food assistance increases the likelihood of food insecure patients to successfully complete their adjuvant therapy.
Short-term oncologic outcomes of an Enhanced Recovery After Surgery protocol for colorectal cancer patients in a community hospital setting
K. Cole 1, M. Toland 2
1Portland Surgical Associates, 2Tufts University School of Medicine
Background: Enhanced recovery after surgery (ERAS) protocols for colorectal surgery have been shown to reduce hospital length of stay and are hypothesized to decrease surgical stress. This may benefit colorectal cancer patients by limiting immune compromise and reducing time to adjuvant chemotherapy initiation, improving survival.
Methods: 49 patients undergoing partial colectomy for colorectal carcinoma were treated using an institution-specific ERAS pathway. Patient and surgery characteristics as well as time to chemotherapy administration were abstracted from a prospective database.
Results: Average patient age was 63 years and 59% were female. Stage breakdown is as follows: 3 patients (6%) stage 0, 16 patients (32%) stage I, 14 patients (29% stage II, 10 patients (20%) stage III, 6 patients (12%) stage IV. The most common operation was right hemicolectomy in 27 patients (55%), and 44 of 49 procedures (90%) were performed laparoscopically. Median length of stay was 3 days. 43 of 49 patients (88%) had ≥12 lymph nodes retrieved, and the average lymph node retrieval was 20. Surgical resection margins were negative in 47 of 49 patients (96%). 15 patients (31%) received post-op chemotherapy. Average time to chemotherapy was 38 days post-op. 5 of 15 patients (33%) started treatment in fewer than 30 days.
Conclusions: Use of an ERAS protocol for colorectal cancer surgery in our community hospital produced excellent short-term surgical outcomes and a favorable time interval to initiation of chemotherapy. ERAS protocols applied on a larger scale may demonstrate improved long-term outcomes for colorectal cancer patients.
Professional Practice Environment of Nurses in Community Ambulatory Oncology Settings: An Exploratory Analysis
E. Dann
Dana-Farber Cancer Institute
Background: Providing care to patients with cancer is very complicated and requires strong interdisciplinary collaboration to be able to provide high-quality care. A positive professional practice environment (PPE) is vital to assure the safety of patients and staff, delivery of excellent patient care, and staff satisfaction. PPEs have been linked to patient outcomes and the work setting of nurses for decades. Favorable practice environments are associated with less emotional exhaustion of staff, improved staff safety, less staff turnover, increased staff and patient satisfaction, increased patient safety, and better patient outcomes. PPEs have been explored in a variety of healthcare settings.
Methods: The purpose of this doctoral project was to describe oncology nurses’ perceptions of their PPE in the community ambulatory oncology setting. A similar study with nurses was completed at a large academic oncology center. The same survey tool (The Practice Environment Scale of the Nursing Work Index (PES-NWI), Safety Organizing Scale (SOS), and other items to assess nurse outcomes) was used in this DNP project, but the current study focused specifically on the community ambulatory oncology setting.
Results: The results of the survey were generally very favorable and aligned to the previous study at the same institution. Although there were no statistically significant results, this project provided meaningful data for leadership to respond to.
Conclusions: The evaluation of the PPE of ambulatory oncology nurses is essential so that leaders can understand how to support favorable nurse outcomes and foster an environment for nurses to provide high-quality care.
Lung cancer screening at a community hospital in eastern and northern Maine.
A. Harb 1, G. Keller 2, J. Klemperer 2
1Eastern Maine Medical Center Cancer Care, 2Department of Thoracic Surgery, Eastern Maine Medical Center
Background: The National Lung Screening Trial (NLST) published in 2011 demonstrated a cancer specific mortality benefit in current and former smokers who were screened with yearly low-dose chest CT (LDCT) compared to chest x-rays.
The goal of our work is to analyze the outcomes of LDCT at a community Hospital and see if the results can be replicated in a community setting.
Methods: It is a retrospective review of all the LDCT done at Eastern Maine Medical Center between July 2014 and June 2018. All the patients screened participated in shared decision making, they also must have met specific criteria (asymptomatic current smokers between the ages of 55 and 77, former smokers who stopped smoking less than 15 years ago).
Results: 1250 scans were performed. 30 scans showed highly suspicious findings (2.4%). 26 had biopsy proven lung cancer and 4 had a presumed malignancy but refused further work-up. 16 had stage I, 3 had stage II, 5 stage III and 2 stage IV. 14 underwent a surgical resection, 3 had SBRT, 8 were treated with chemotherapy and radiation and one refused treatment.
Conclusions: Our results were consistent with those previously published in the literature. In a community-based model in Eastern and Northern Maine, a lung cancer screening program was able to identify about 73% of patients in early stages (19/26 patients with stages I/II). We hope that these outcomes would translate into increased cure rates and decrease mortality.
Patient Reported Outcomes in Hematopoetic Stem Cell Patients: A Pilot Project
C. Hayes, K. Meehan, A. Tosteson, D. McKenna, K. Wilcox, L. Root, C. Reed
DHMC - Norris Cotton Cancer Center
Background: Patient-reported outcomes (PROs), including symptoms and quality of life (QOL) measures capture the patient-centered experience of hematopoietic cell transplantation (HSCT). They provide the opportunity to enhance patient-provider communication and allow for symptom intervention. PROMIS-29 is one of the NIH developed and validated tools for collecting PROs. It examines 7 domains: physical function, anxiety, depression, fatigue, sleep disturbance, social participation, and pain interference. We have piloted its use in the HSCT patients.
Methods: Twenty five patients both pre- and post- hematopoietic stem cell transplantation (allogeneic, n = 14; autologous, n = 11) were surveyed using the PROMIS 29 scale. Patients ranged from 4.6 months pre-transplant to 5.8 years post-transplant. The patients completed the PROMIS-29 survey in the waiting room prior to their scheduled appointments.
Results: The top three reported concerns were decreased physical function (38.5%), fatigue (30.8%) and pain (20%). Fatigue remained a prominent complain with patients commonly reporting moderate to severe fatigue post-transplant. Strikingly, patients reported fatigue commonly whether they were within a year of their transplant or several years out from their transplant.
Conclusions: PROs, such as PROMIS-29, provide valuable insight into the HSCT patient experience and QOL. Our pilot data, demonstrates that these symptoms can persist long after the patient has been cured of their hematologic malignancy. We are broadening the implementation of this survey to capture all HSCT recipients. We plan to use the results to enhance communication at our outpatient visits. We also plan to use the aggregated data to define areas for future intervention to help our patients cope following transplantation.
 Best Practice Medication Reconciliation in the Outpatient Setting
Best Practice Medication Reconciliation in the Outpatient Setting
M. Hession
Center for Cancer Care at Exeter Hospital
Background: Medication safety is a focus of the Joint Commission’s National Patient Safety Goals and research supports improved medication reconciliation as a strategy to reduce medication errors and adverse drug events. In an outpatient specialty clinic where patients are routinely considered for high-risk therapies, a consistent medication reconciliation process is essential for patient safety and positive health outcomes.
Methods: Based on a gap analysis between evidence-based and current practice, a quality improvement intervention was implemented to increase patient engagement in the medication reconciliation process. A reminder prompt was added to automated appointment notification calls and staff provided verbal cues to patients along with a printed copy of the medication list for review during the check-in and rooming process. A report was created to capture whether medication reconciliation was completed at the same time as provider-patient visits, and rates of reconciliation completion were calculated.
Results: Prior to implementation of this project, medication reconciliation completion rates were calculated at an average of 35.6% over the three months prior. During the six-week intervention period, reconciliation rates improved in the range of 4.4-10.7% over that of the pre-intervention average rate. Medication list completeness and accuracy, however remain a challenge.
Conclusions: Increased patient engagement showed a positive effect on medication reconciliation completion rates in the outpatient setting but did not surpass the goal of at least 50% reconciled. Further interventions, including staff training to improve competency in accurate medication reconciliation is warranted.
Utility of Breast Cancer Index (BCI) in the clinical practice
S. Kodali, K. L. Dittus, E. Tipirneni
University of Vermont
Background: Extended endocrine therapy (EET) greater than 5 years in early stage HR+ BC patients has shown benefit. However, EET is associated with side effects and there is no standard way to determine which group of patients would derive benefit. BCI is a validated biomarker test that incorporates 2 distinct genomic assays and is prognostic/predictive.
The objective of this study is to assess patient characteristics, pathologic features and patient preferences with regards to extending endocrine therapy after reviewing BCI results.
Methods: BCI has been offered to patients deemed appropriate since fall of 2015. We performed a retrospective chart review on early stage HR+ BC patients from Jan 2016 to Jan, 2017. We identified 25 cases on whom BCI was submitted.
Results: Median age was 67 years. Majority of the patients were stage IA (64%). The tumors were moderately differentiated in 56% and HER2+ in 12%. Median tumor size was 1.4 cm. The primary reason the test was sent was poor tolerance to the ET in 76%. In Lymph node negative patients, BCI identified 42% as high risk, 52% as low risk for late recurrence and 32% who derive high benefit from EET. In the entire cohort, 40% were categorized to as having a high likelihood of benefit from EET. Based on results of the BCI, 70% elected to continue EET.
Conclusions: TBCI is a reasonable test to consider in early stage HR+ BC patients especially in patients with poor tolerance to ET and might help in decision making for EET.
Association of behavioral counseling with sun protection use in adults: results of a survey
K. Lehmann, K. Cole
Portland Surgical Associates
Background: Skin cancer, though preventable, remains the most common form of cancer in the United States. Evidence reviews conducted by the US Preventative Services Task Force (USPSTF) show that behavioral counseling for fair-skinned individuals older than 24 years may result in an increase in use of sun safety measures.
Methods: Surveys were provided to patients seen for all diagnoses for a period of one month in our outpatient surgical office. Data collected included: age, gender, prior skin cancer diagnosis, frequency of sun protection measure use, prior behavioral counseling regarding sun protection use, and involvement of a dermatologist in the patient’s care.
Results: 106 patients completed the survey. The average age was 56 and 58% were female. 49 (46%) stated their PCP had counseled them regarding sun protection (sunscreen, hat, protective clothing, etc.). In this group, 4 patients (8%) reported “never” using sun protection, 20 (41%) “sometimes”, 14 (29%) “usually”, and 11 (22%) “always”. In the group of 53 patients who reported no counseling, 13 (25%) “never” use sun protection, 14 (26%) “sometimes”, 15 (28%) “usually”, and 11 (21%) “always” (p=0.03 for comparison of the two groups with respect to “never” vs. all other responses combined). Patients in the PCP counseling group were more likely to have had a prior skin cancer diagnosis and to have a dermatology consultant involved in their care.
Conclusions: Counseling from a primary care physician was associated with a lower chance of “never” using sun protection in a small sample of our adult patient population.
A survey of hospice and palliative care nurses’ and holistic nurses’ perceptions of spirituality and spiritual care
E. McGrath 1, J. Lukovsky 2, M. A. Beauchesne 3
1DHMC - Norris Cotton Cancer Center, 2New York Presbyterian, 3Northeastern University
Background: The provision of spiritual care is stipulated in professional practice guidelines and mandated in nurses’ ethical codes. Still, a gap exists regarding essential training in spiritual conversation and assessment, leaving some health care providers feeling uncomfortable when assessing/meeting spiritual support needs. The purpose of this study was to assess Hospice and Palliative Nurses’ (HPN) and Holistic Nurses’ (HN) perceptions of spirituality and spiritual care. It was assumed that the Standards of Care for HPN and HN stipulate that spiritualty is addressed within the framework of their specialties and provide education for spiritual care, thus making these nurses proficient in providing spiritual care.
Methods: This exploratory, descriptive study utilized a web-based survey to measure perception of spirituality and spiritual care giving using a validated tool. A convenience sample was recruited from members of the Hospice and Palliative Nurses Association (HPNA) and the American Holistic Nurses Association (AHNA) (n=250)
Results:
•64.85% able to meet patient’s spiritual needs as a regular part of practice
•80.50% address patient’s spirituality every time they work
•65% of the nurses felt that they were able to meet spiritual needs of their patients by delivering support and reassurance (65.5%), by listening to patients, and exploring their fears and anxieties (68.2%)
Qualitative themes emerged:
•Use of presence and therapeutic listening
•Finding meaning and purpose in life
•Teamwork
•Exploration of feelings
•Respect and support
Conclusions: This study adds to an emerging body of evidence suggesting that training in spiritual care should be an important component of the foundational nursing curriculum and continuing education
Fighting Two Battles: An Analysis of Caregivers’ Out-Of-Pocket Costs Following Transplantation
J. M. Meehan, K. Wilcox, L. Root, E. Busnach, B. Labrie, D. McKenna, J. M. Hill
Dartmouth Hitchcock Medical Center
Background: During treatment for cancer, caregivers of cancer patients face significant out-of-pocket costs.
Methods: 7We created a clinical trial that utilized weekly surveys to assess the out-of-pocket caregivers’ costs, in the four weeks after discharge following an autologous stem cell transplant. Caregivers identified the amount of money spent or lost in each of the following categories: missed work, lost wages, travel (mileage, time, fuel, or tolls) and additional costs (prescriptions, co-payments, or accommodations).
Results: Twenty-nine caregivers consented to the trial. In almost every instance, the caregiver was the spouse of the patient. The number of weekly doctor visits was 3.4 (mean; range: 3-4).Of the 29 caregivers, 14 caregivers lost 21.3 hours of work (mean; range: 2-32) with an associated loss of $534 in wages (mean; range: $30-$1050). Over the course of the four weeks, the caregiver traveled 496.3 miles (mean; range: 36-824) and spent 9 hours 52 minutes on the road (mean; range: 1hr30mins-17hr15mins). Furthermore, caregivers spent $60 for travel-related expenses (mean; range: $0-$144), $24 for food (mean; range: $0-$105) and $45 for additional costs (mean; range: $0-$222), including co-payments, accommodations etc. For the 29 caregivers, the total out-of-pocket costs were $369 per caregiver (mean; range: $36-$1113), with the majority of costs derived from lost wages.
Conclusions: In summary, caregivers experience significant out-of-pocket costs in the 4 weeks following discharge from the hospital, which adds to the stress of a complicated transplant process.
m-Palliative Care Link: A mobile application to improve assessment of and communication regarding symptom control among late-stage Tanzanian cancer patients
S. Miesfeldt1, R.Morse2, S. Sagan3, K. Lambden3, E. Quinn3, H. Mahuna4, M. Ngoma5, T. Ngoma5, B. Mushi5, Y. Xian Ho2
1MMC Cancer Institute, 2DaVinci Usability Inc., 3Dimagi, Inc., 4Ocean Road Cancer Institute, 5Muhimbili University of Health and Allied Sciences
Background: Access to effective palliative care is a Tanzanian public health priority, with recognized need for innovative community-based solutions. Mobile technology holds promise in this regard; however resources are limited. The study goal is to develop and pilot-test a mobile device-based symptom assessment/control communication system (m-Palliative Care Link; mPCL) to: extend access to a limited number of palliative care specialists (hereafter, specialists); improve symptom-control information exchange between specialists, patients, and local health workers (LHWs); reduce late-stage cancer patient symptom burden.
Methods: mPCL is based on an existing patient/caregiver-directed, symptom-based, 10-item Palliative care Outcome Scale, adapted for mobile delivery. In partnership with Tanzanian specialists, we created a secure smartphone application-based communication system (mPCL) for remote symptom assessment and communication among specialists, patients/caregivers, and LHWs (i.e., user groups). Through hands-on observation/feedback and surveys, user group representatives provided application ease-of-use feedback.
Results: Twenty participants (representing all user groups) completed usability tests. Participants were able to successfully use the application, and felt that it would be very helpful to them. mPCL usability recommendations were collected to include: redesign data collection form to better fit communication needs, simplify the patient/caregiver application, broaden access to clinical data. These findings are being used to improve mPCL design for planned field test later this year.
Conclusions: As mPCL is reliant on a freely available, secure mobile application and existing personnel, it promises to: be sustainable and transferable to Tanzanian adults and children with a number of chronic conditions; serve as a model system for other low-income countries.
Malnutrition screening: A Screening Tool for Outpatient Oncology Patients
J. B. Mills
Dartmouth Hitchcock Medical Center
Background: The provision of adequate nutritional care in outpatient cancer centers was the focus of a 2016 NAS Workshop, "Assessing Nutrition Care in Outpatient Oncology". Here we report our internal project evaluating ongoing documentation of a malnutrition screening tool (MST) at 3 national cancer centers(CC).
Methods: Screening scores from a validated 2 question MST scale were entered into the EMR. Questions probe for : 1). unintentional weight loss; and 2)eating poorly because of a decreased appetite. A score of greater than or equal to 2 indicated nutrition risk. De-identified oncology clinic visit data were examined monthly to assess MST utilization and scores for radiation and medical oncology patients across the CC's.
Results: Approximately two-thirds (67%) of unique medical oncology patients that visited the CC's had documented MST data with 9% (n=144,129)scoring at nutritional risk. MST completion rates were higher in radiation oncology clinics secondary to staff education. Of those that had a valid MST score in radiation clinics, 13% (n=23,202) of MST scores indicated nutritional risk.
Conclusions: The MST is a valid malnutrition screening tool for outpatient oncology patients, yet this is not uniformly being utilized nationally. Consistent use of the MST in the electronic medical record and leveraging data on utilization are needed to inform staff compliance, consistency in care, future dietitian staffing patterns, cost/benefit analysis, and health outcomes for oncology patients.
Association of rurality with survival and receipt of treatment in early-stage non- small cell lung cancer patients in the United States
C. Nicoli, B. L. Sprague, N.H. Lester-Coll
Larner College of Medicine at the University of Vermont
Background: Lung cancers are the leading cause of cancer-related death in the United States. While multiple studies have identified disparities in survival among rural lung cancer patients, this has not been consistent.
Methods: We performed a population-based retrospective study of non-small cell lung cancer (NSCLC) patients from the National Cancer Data Base to assess the impact of rurality on survival. Median overall survival (OS) was compared using the log-rank (LR) test; the Cox proportional-hazards model was used in multivariable modeling.
Results: We identified 840,566 patients diagnosed with primary NSCLC between 2005 and 2015. Of these, 18.7% resided in rural areas. Rurality was associated with greater proportions of males, white patients, and those from areas with lower median annual income and education (χ2 p < 0.001). OS in all-stage NSCLC statistically differs among rural (10.18 months) and non-rural patients (11.24 months), a small disparity of 1.06 months (χ2 p <0.0001). Patients with stage I NSCLC have a survival disparity of 11.07 months (rural OS=50.30 months, non-rural OS=61.37 months, p <0.0001). A higher proportion of stage I rural patients (53.1%) did not receive guideline-concordant management (lobectomy or stereotactic body radiation therapy), relative to 49.8% of non-rural patients. A 3.1% greater proportion of rural stage I patients had one or more comorbidities (χ2 p<0.001). In multivariable modeling, rurality remained an independent risk factor for death in stage I and all-stage NSCLC.
Conclusions: Rurality is independently associated with a substantial survival disparity in stage I NSCLC. These rural patients have more comorbidities and less often receive guidelines-concordant management.
Palliative care utilization in patients with pancreatic cancer: A retrospective chart review at an academic tertiary-care center
V. Pauer1, E.B. McGrath2
1Saint Anselm College / Dartmouth-Hitchcock Norris Cotton Cancer Center, 2Dartmouth-Hitchcock Norris Cotton Cancer Center
Background: Pancreatic cancer is the third most common cause of cancer-related deaths, with a 5-year survival rate of 8%. Pancreatic cancer patients experience clinically significant distress related to poor prognoses, which can be alleviated through early palliative care involvement. This study aimed to understand the current pancreatic cancer patient population at the Norris Cotton Cancer Center (NCCC), assess the current utilization of palliative care, and examine the relationship between patient distress and palliative care involvement.
Methods: A retrospective electronic medical record review was conducted to collect demographic and care-related data from pancreatic cancer patients (n=105) who received oncology treatment at the NCCC from April 2016 to June 2018. Patient data was recorded without patient identifiers on an Excel sheet and statistically analyzed for relationships.
Results: The following demographic profile was determined for patient population at the NCCC: 58% male, 76% >60 years old, 99% Caucasian/White, 50% married/cohabitated, 64% retired, 75% on Medicare, and 54% initially diagnosed with Stage IV cancer. Additionally, patients categorized by stage of cancer received initial palliative care consults at the following rates: 33% Stage I, 67% Stage II/III, and 56% Stage IV. Forty-one percent of patients were screened for distress, and 63% of those screened had clinically significant distress. Eighty-nine percent of patients with clinically significant distress were seen by palliative care.
Conclusions: Pancreatic cancer patients experience high rates of distress related to their diagnoses and would benefit from palliative care consults. Distress screening and palliative care involvement must be priorities in the plan of care for pancreatic cancer patients.
Realizing the Benefits of an Integrated Care Model through the “white bagging” process in a community based Oncology Clinic Pharmacy.
D.Raymond, P. Skeffington, B. Gomes, E. Fortier, H. McCarthy
Southcoast Centers for Cancer Care
Objective: To assess the benefit of our current Integrated Specialty Pharmacy services within our organization as it applies to infusion and injectable oncology medications.
Background: Increased development of specialty pharmaceuticals (to treat rare, chronic and complex medical conditions), resulted in our Health System developing its own specialty services. The SCCC clinic pharmacy retrospectively reviewed current processes for white bagging. We examined coordination of patient care, procurement and distribution of specialty medications and the benefits of cost containment to the organization.
Methods: The pharmacy retrospectively reviewed EMRs of patients using specialty pharmacy injectable or infused medications over a two year period from 6/2016 to 6/2018. The following data was reviewed: medication administered, procurement pharmacy, and cost analysis.
Results: Over two years, $600,000 was saved by the clinic (cost avoidance) in drugs that were given in the oncology clinic but billed through specialty pharmacies (based on 340b pricing). In this retrospective analysis, 50% of patients used Southcoast Specialty Pharmacy versus preferred network. Just in time dispensing added to patient satisfaction results that are stellar.
Conclusions: SCCC offers a comprehensive system to provide specialty pharmaceuticals. Thorough review showed areas for improvement. Program development could benefit from improved interdisciplinary approach, quality metrics and opportunities for revenue growth.
Notes: Hypothesis
Oncology patients and SCCC benefit from the Integrated Care Model (ASHP) providing continuity of care, coordination of white bagging, and cost containment.
Maine’s impact cancer network enables statewide collaboration
A. Sheikh, H. Drake
Maine Cancer Foundation
Background: In 2015, funding for Maine’s Cancer Consortium ceased. To preserve a statewide cancer coalition, Maine’s Impact Cancer Network (the Network) was created. Since 2016, the Network has served as the only statewide convener for Mainers seeking to reduce the burden of cancer in Maine.
Methods: The Network utilizes a collective impact model to implement its mission to reduce the impact of cancer on individuals and communities in Maine through collaboration and systems improvement. Qualitative data from 670 Mainers, along with quantitative state data, and the guidance of a leadership roundtable and data team, were used to create a common agenda. The Network’s common agenda includes healthcare system collaboration, access to care, education about cancer prevention, detection and treatment, advocacy and survivorship. It is implemented through seven task forces.
Results: The network now has 450 members and task forces have executed three collaborative projects. 64% of Network members stated that participation in a task force has fostered relationships with other members. Impact of the Network will be determined using cancer registry data in addition to analysis of more nebulous measures such as collaboration and system change.
Conclusions: Despite common goals, many Maine cancer care professionals often do not work together due to systemic barriers. The Network has successfully utilized a collective impact framework to unite stakeholders to address shared priorities. Continued funding and participation in collaborative cancer work will be essential for improved statewide cancer care.
Notes: Both authors, Aysha Sheikh and Heather Drake, have no conflicts.
Feasibility of opioid risk screening in an oncology clinic: A pilot study
L. Teulings, M. Storms, K. Beloin, P. LeBlanc, L. Pellegrino, K. Shirai
Norris Cotton Cancer Center, DHMC
Background: Opioids are considered to be integral to treatment of cancer related pain, yet there is a paucity of data on the prevalence of opioid misuse in this population. Furthermore, there are no established guidelines regarding assessment and management of opioid misuse in cancer patients. A feasibility pilot study to screen for opioid risk was conducted in two clinics in a NCI cancer center.
Methods: The Opioid Risk Tool (ORT), a validated eight question screening tool, was administered by a registered nurse to patients in thoracic and head and neck oncology clinics at a pre-treatment visit to assess for feasibility of use. The score was recorded by the nurse in the electronic medical record.
Results: Feedback from the nurses administering the survey stated it was quick and easy to administer. Of the 20 patients screened over a one month period, 35% were moderate to high risk (4 from thoracic and 3 from head and neck). A personal history of substance abuse (alcohol, prescription and/or illicit drugs) was a common risk factor in 6 of the 7 moderate to high risk patients.
Conclusions: Use of the ORT is feasible in an oncology clinic. Preliminary data from this small sample suggests that risk factors for opioid misuse exist among cancer patients. Future plans are to expand screening using the ORT across various disease groups at the cancer center in order to better understand point prevalence and risk factors of opioid misuse among this population.
Interim analysis of the Impact of a Serious Illness Communication Skills Training in Hematology-Oncology Fellowship on Discussion and Documentation of Patient Values and Goals
G. Wasp1, T. P. Meehan Jr3, A. M. Cullinan3,4, A. M. Donovan2, M. D. Chamberlin2, C. A. Hayes2, A. E. Barnato5, M. T. Vergo3,4
1 DHMC - Norris Cotton Cancer Center, 2 Division of Hematology and Oncology, Dartmouth-Hitchcock Medical Center, 3 Geisel School of Medicine at Dartmouth, 4 Division of Hospice and Palliative Medicine, Dartmouth-Hitchcock Medical Center, 5The Dartmouth Institute of Health Policy and Clinical Practice, Geisel School of Medicine at Dartmouth
Background: Providing effective training in serious illness communication is an explicit goal of hematology-oncology fellowship. We conducted a single center implementation study of a validated communication skills training program delivered to all fellows and a selected group of oncology faculty “coaches.” This abstract presents interim (six month) analysis.
Methods: Intervention - The training was centered on a conversation script/checklist, Serious Illness Conversation Guide (SICG)©. Fellows underwent seven didactic sessions, two simulated patient encounters, and anticipated ongoing feedback from trained faculty. Assessment – We assessed learner confidence at baseline and four months later. An independent rater scored simulated encounters at one and four months from intervention start. We electronically assessed incorporation of the SICG into fellow’s notes every two months.
Results: Eight fellows completed training, seven participated in both simulated patient encounters (88%). Primary outcome – At six months, two of six study-group fellows (33%) documented SICG in a clinical encounter. Secondary outcomes –At three months, six to seven fellows (75-88%) agreed they were more confident in eight different SICG related competencies (all assessed). In simulated patient encounters, fellows’ median score was 13 out of 16 SICG specific items at both time points (Z = -1.13, p = 0.26); mean overall communication skills increased from 3.6 and 5 (out of 9) (Z = -2.20, p = 0.028).
Conclusions: Improvements in self-efficacy and stable or improving simulated encounter scores suggest we are improving and retaining fellows’ communication skills. Future work should focus on barriers to documentation of the SICG in clinical notes.
Increased Health Care Costs Associated with Venous Thromboembolism in Patients with Malignant Glioma
H. Wright, C. Holmes, A. Thomas
University of Vermont Medical Center
Background: Venous thromboembolism (VTE) occurs frequently in patients with malignant glioma (MG), with estimates ranging from 20-35% during treatment. The development of VTE is associated with increased morbidity. The aim of this study was to assess the healthcare burden associated with VTE in patients with MG.
Methods: A retrospective chart review of patients with MG at the University of Vermont Medical Center was conducted from 2009-2017. Sixty-seven patients, age greater than 18, with a histologic diagnosis of MG (WHO grade III-IV) were assessed. The number of emergency room (ER) visits and inpatient hospitalizations with the associated costs were collected and analyzed.
Results: Eighteen of 57 patients developed VTE (27%), and all were placed on therapeutic anticoagulation. 50% developed complications related to anticoagulation. Patients with VTE spent more time in the hospital with an average of 16.6 inpatient days as compared to those without VTE (10 inpatient days; p=0.012). Those with VTE experienced 3.94 ER visits compared to 1.84 visits by patients without VTE (p=0.003). The average primary total cost (ER visits + hospitalizations) was 26% higher in patients with VTE (VTE: $48,863; no-VTE: $35,948).
Conclusions: The development of VTE in patients with MG increases inpatient admissions days and incurs additional pharmaceutical costs related to anticoagulation. This study represents the first assessment of VTE-associated health care burden specific to primary brain cancer. VTE may be a preventable complication in MG and further studies are needed to investigate safe prevention strategies.
Abstract Subgroup: Treatment, chemo regiments, treatment paradigms, etc.
Palbociclib Plus Endocrine Therapy (ET) in Hormone Receptor–Positive (HR+), Human Epidermal Growth Factor Receptor 2–Negative (HER2–) Advanced Breast Cancer (ABC): Updated Summary of the PALOMA Clinical Program
E. Bananis1, R. S Finn2, N. C. Turner3, A. Mori4, K. Puyana Theall1, D. Ray Lu1, A. Schegoleva1, V. Diéras5, D. J. Slamon2, M. Cristofanilli6
1 Pfizer Inc, 2 David Geffen School of Medicine at UCLA, 3 Royal Marsden Hospital and Institute of Cancer Research, 4 Pfizer S.r.l., 5 Centre Eugène Marquis, 6 Robert H. Lurie Comprehensive Cancer Center, Feinberg School of Medicine
Background: Palbociclib is a cyclin-dependent kinase 4/6 inhibitor approved for treatment of HR+/HER2– ABC. Results from PALOMA-1 and interim results from PALOMA-2/-3 have been previously published; updated progression-free survival (PFS) data from PALOMA-2/-3 are presented.
Methods: Palbociclib was studied in 3 clinical trials in HR+/HER2– ABC patients. Methodology for the trials has been published previously; primary endpoint in each was PFS.
Results: In the open-label PALOMA-1 trial (n=165), median PFS (mPFS) was 20.2 (95% CI: 13.8–27.5) vs 10.2 (5.7–12.6) months for palbociclib+letrozole vs letrozole, respectively; HR 0.49 (95% CI: 0.32–0.75; P=0.0004). In the double-blind PALOMA-2 trial (n=666), mPFS was 27.6 (22.4–30.3) vs 14.5 (12.3–17.1) months for palbociclib+letrozole vs letrozole, respectively; HR 0.56 (0.46–0.69; P<0.000001; May 2017 data cutoff). In the double-blind PALOMA-3 trial (n=521), mPFS was 11.2 (9.5–12.9) vs 4.6 (3.5–5.6) months for palbociclib+fulvestrant vs fulvestrant; HR 0.50 (0.40–0.62; P<0.000001). In PALOMA-2/-3, palbociclib+ET demonstrated PFS benefit vs ET alone in patients with visceral (including lung and liver), nonvisceral, and bone-only metastases. Common adverse events occurring in >10% of patients receiving palbociclib+ET were neutropenia, infections, leukopenia, fatigue, nausea, stomatitis, anemia, alopecia, diarrhea, and thrombocytopenia.
Conclusions: Results from the PALOMA program demonstrate that palbociclib+ET significantly prolongs PFS vs ET alone, with a generally tolerable safety profile. Palbociclib+ET should be considered as a first-line and later lines treatment option for HR+/HER2- ABC.
Notes: Funding: Pfizer (NCT00721409; NCT01740427; NCT01942135).
Reused with permission from FLASCO. This abstract was presented at the 2018 Spring Session. All rights reserved.
Treatment Intensity and Length of Stay in Elderly Acute Leukemia Patients
A. Baronner, C. Hayes
Dartmouth Hitchcock Medical Center (DHMC)
Background: The optimal treatment of elderly patients with acute leukemia remains a difficult clinical scenario. Elderly patients have lower rates of remission and more aggressive variants of the disease. They also experience increased morbidity and early mortality with more intensive therapies. Hospital length of stay may represent increased morbidity associated with treatment.
Methods: We conducted a retrospective review of all patients with acute leukemia discharged from the DHMC inpatient hematology oncology service from 1/2017 to 5/2018 (n=43). Ages ranged from 21 to 89. 23% had a diagnosis of ALL and 77% AML. 58% (25/43) patients were age 60 or greater. Length of stay (LOS) was assessed for each of the patients. Treatment was classified as standard intensive (SI) chemotherapy verses reduced intensity (RI) chemotherapy.
Results: The average LOS for the study population was 48.3 days. Of the elderly acute leukemia patients, 40% (10/25) received SI verses 60% (15/25) received RI regimens. Average LOS in elderly patients in SI group was 58.1 days verses 13.6 days in RI group. Mortality was 40% in SI group and 46% in RI group.
Conclusions: Treatment decisions in elderly acute leukemia patients have important implications. Of the elderly patients examined, more received RI chemotherapy. RI chemotherapy was associated with a shorter LOS compared to patients receiving SI chemotherapy and mortality was similar in both groups. Thus, RI treatments may offer patients less time in the hospital and better quality of life.
Review of first line treatments for stage I non-small cell lung cancer patients
A. J Harb, M. Soliman, A. D. Curtis
Eastern Maine Medical Center Cancer Care
Background: A review of all stage I non-small cell lung cancer (NSCLC) cases treated between 2010 and 2015 at Eastern Maine Medical Center (EMMC), was conducted to investigate first line treatments and associated outcomes.
Methods: There were 401 patients treated during this time period comprised of 264 (65.8%) stage IA and 137 (34.1%) stage IB patients. First line treatment was primarily one of two modalities; surgery (237, 59.1%), or stereotactic body radiation therapy (SBRT) (88, 22.1%). The remaining patients were treated via other radiation methods, chemotherapy, or in various combinations of these. Local and distant recurrences of lung cancer or metastases thereof were recorded at an 18 month interval post first line treatment. Only patients with at least 18 months of follow up contact after first line treatment were included in the analysis of recurrence rates (293 patients).
Results: Recurrence rates (local+distant), were determined to be 16.2% and 31.0%, for surgery and SBRT treatments respectively (OR: 2.32[95% CI: 1.18-4.57] p<0.02). Among patients treated with SBRT, only 8 of 58 patients had a recurrence within the initial treatment field (local recurrence) (13.8%). Ten patients receiving surgery had recurrence in the same lung laterality as the primary tumor (5.2%).
Conclusions: Many factors play a role in the difference between recurrence rates in surgery and SBRT. Age and Charlson comorbidity scores of patients who received SBRT were higher compared to those undergoing surgery indicating a self-selection of patients with poorer health baseline for those receiving targeted radiation (p=0.0003 and p=0.00005, respectively).
Review of the treatment milestones for stage I non-small cell lung cancer
A. J Harb, G. A. L’Italien, N. C. Bullion, A. D. Curtis
Eastern Maine Medical Center Cancer Care
Background: Treatment milestones were reviewed for all stage I non-small cell lung cancer (NSCLC) patients treated at Eastern Maine Medical Center (EMMC), from 2010 to 2017. Cases in which the initial time to treatment (TTT) exceeded 6 months were considered non-typical and were excluded from analysis, resulting 508 cases for this time period.
Methods: Analysis demonstrated that patients primarily took one of three different pathways in the diagnostic process. This process typically involved a CT scan, PET scan, and a biopsy. The three primary diagnostic pathways were: CT-PET-Biopsy (28.9%), PET-CT-Biopsy (13.9%), or CT-PET (no biopsy) (38.2%), accounting for 81% of all patients. The primary mode of treatment for most patients was surgery (62.0%). Patients not eligible for surgery receive stereotactic body radiation therapy (SBRT) (24.8%).
Results: The three step diagnostic pathways did not significantly differ from each other in terms of TTT (p=0.59). The two step diagnostic pathway of CT-PET was shorter on average than the CT-PET-biopsy pathway (p=0.013). Patients undergoing surgery experienced a shorter average TTT compared to patients receiving SBRT (p=0.00000001). There was an increase in TTT among patients diagnosed at other facilities and referred to EMMC for treatment (p=0.044), while EMMC patients TTT remained stable over the time course.
Conclusions: Differences in TTT between treatment modalities is likely related to patient comorbidities and additional time needed for radiation planning and simulation. The goal of this study was to identify the current treatment milestones and related wait times in an effort to further optimize patient care.
Review of treatment milestones for head and neck cancer at a regional rural cancer center
A. J Harb, G. A. L’Italien, N. C. Bullion, A. D. Curtis
Eastern Maine Medical Center Cancer Care
Background: Treatment milestones for head and neck malignancies were reviewed for all head and neck cancers treated at Eastern Maine Medical Center (EMMC), from 2013 to 2017. Cases included cancers of the oral cavity, oropharynx, larynx, and hypopharynx. Nasopharyngeal, parotid/salivary glands and thyroid malignancies were excluded from this review. There were total of 294 cases diagnosed and treated during this time.
Methods: Analysis showed that patients took multiple pathways through the diagnostic and treatment process. Major diagnostic milestones included: biopsy, CT scan, and PET scan. We identified 14 distinct pathways taken by these patients, with 73.5% of patients taking one of four pathways; CT-Biopsy-PET, Biopsy-CT-PET, Biopsy-CT, or Biopsy-PET. The primary mode of treatment was a combination of chemotherapy with radiation (concomitant) with 53.4% of patients receiving this therapy.
Results: There were 35 unique pathway sequences taken through the diagnostic and treatment process by 294 patients. Thirteen of those pathways are unique to a single patient. The 3 step diagnostic pathway duration of biopsy-CT-PET is an average of 9.5 days shorter than CT-biopsy-PET (23.6 and 33.1 days respectively, p=0.0004). Increased number of diagnostic steps (2 to 3) resulted in longer time to treatment (TTT) (40.5 and 54.4 days respectively, p=0.00005). TTT was stable over the 5 years examined (p=0.38) and was not affected by the ultimate modality of first treatment (p=0.60).
Conclusions: The goal of this study was to identify the current treatment milestones, pathways, and related wait times, in an effort to identify the barriers and further optimize patient care.
Early Experience with Vyxeos: Clinical and Practical Considerations
C. Hayes, K.Karkowski
Dartmouth Hitchcock Medical Center
Background: In older patients, there is a higher incidence of treatment related AML (t-AML) and AML with myelodysplasia-related changes (AML- MRC), both of which carry a poor prognosis. In 8/2017, Vyxeos, a liposomal formulation of daunorubicin and cytarabine, received FDA approval for use in patients with t-AML or AML-MRC because of improved overall survival when compared to traditional therapy.
Methods: We conducted a retrospective review of all patients with AML treated with Vyxeos at DHMC starting from January to August of 2018 (n=6). All patients had either t-AML or AML-MRC, were over the age of 60, and were potential candidates for allogeneic stem cell transplant in the future. Length of stay (LOS), duration of nadir, treatment outcomes, and treatment cost were calculated for all patients.
Results: The average LOS for those treated as an inpatient was 61 days (n=4). Both of the patients who received induction as an outpatient were subsequently admitted for neutropenic fever (6 days, 71 days). 4/6 of patients are still planned for allogeneic transplant. An initial experience with pegfilgrastim support seems to have shortened the nadir and resulted in a decreased cumulative LOS.
Conclusions: Our institution’s experience with Vyxeos has been mixed. 4/6 patients, all over the age of 65, are progressing towards allogeneic transplant. However, both LOS and duration of nadir have been quite long. This has implications for patients’ quality of life and also for financial stewardship. Consistent early pegfilgrastim support may be a way to shorten nadir, LOS, and decrease hospital costs.
Fluconazole prophylaxis and invasive fungal infections in AML patients receiving induction/consolidation chemotherapy.
S. Kodali, W. Jehangir, D. Douce, R. Cade, E. Umyarova
University of Vermont
Background: AML patients who undergo induction or consolidation experience a period of profound neutropenia during which invasive fungal infections (IFI) are a major complication. At UVM Medical Center, the institutional practice was to give fluconazole during the duration of neutropenia. Fluconazole does not cover Aspergillus and Fusarium, and these patients remain prone to these infections.
Methods: We performed a retrospective chart review on AML adult patients who received induction or consolidation chemotherapy at University of Vermont Medical Center for the past ten years (2006-2016) to determine if fluconazole prophylaxis was sufficient.
Results: We identified 84 patients with AML between 2006-2016 who were given fluconazole prophylaxis to prevent IFI. The median age was 61 (21-91) and 34.5% were females. The incidence of all category IFI was 34% (27% proven, 3.4% probable and 68.9% possible). Mean duration of neutropenia was 41 days in the group that developed IFI. Median time to develop IFI was 46 days after the diagnosis of AML. People who have had underlying lung diseases were noted to be at increased risk for IFI (p= 0.04). There was a trend towards decreased survival among people with IFI with 55% alive at 6 months vs 69% among those without an IFI (p= 0.21).
Conclusions: Given higher incidence of IFI in our AML patients, we have changed our institutional prophylaxis in AML patients from fluconazole to voriconazole in Jan, 2017.
Man vs machine: Efficacy of Neulasta vs Onpro at the University of Vermont Cancer Center
D. Krakauer, D. R Douce, H. Ng, A. Byington, M. A Hinton, M. Wood, H. Rehman
University of Vermont
Background: The granulocyte-colony stimulating factor (G-CSF) pegfilgrastim is administered as an injection in clinic or as an on-body injector (Onpro®) that delivers a standard dose 27 hours after placement. We hypothesized that Onpro® may fail to deliver the G-CSF, potentially resulting in greater rates of neutropenic fever compared to standard injection.
Methods: Patient data on G-CSF use at UVMCC from 1/2016 to 12/2017 were obtained. The primary outcome was development of a neutropenic fever and secondary outcomes included documentation of neutropenia or delays in chemotherapy.
Results: Of 326 patients receiving G-CSF injections, 91 were via Onpro®. The median number of injections per patient was 4. Average age was 58 years, 63.2% were female, average BMI was 27.2 and known stage IV disease was present in 33.8%. Cancer types were 103 breast (31.6%), 44 GI (13.5%), 27 GU (8.3%), 46 lymphoma (14.1%), 31 lung (9.5%), and 75 other (23.0%). 24 patients (7.4%) developed neutropenic fever, of whom 7 (7.7%) received Onpro®, and 17 (7.2%) received in-clinic injections (p=0.86). There was no significant difference in mean age, BMI, sex, proportion of stage IV disease, incidence of neutropenia (p=0.67), or delays in chemotherapy (p=0.49). There was an increased risk of developing neutropenic fever with breast cancer and lymphoma regimens independent of G-CSF delivery method (OR 2.32 [95% CI 1.0-5.36] and OR 2.78 [95% CI 1.08-7.12] respectively).
Conclusions: In a retrospective comparison of Onpro® to standard injection, there was no significant difference in incidence of neutropenic fever, neutropenia, or delays in chemotherapy.
Evolving conditioning for allogeneic stem-cell transplants in the treatment of myeloid malignancies Experience from a Single, Rural-Based Transplant Center
A. P Briand 1, D. McKenna 2, D. R McKenna3, C.H Lowrey3, C. Ann Hayes3, S. C Brighton3, E. A Kimtis3, L. D Root3, K. L Wilcox3, E. Z Busnach3, C. M Coughenour3, K. R Meehan3 , J. M Hill, Jr3
1Department of Medicine, Dartmouth-Hitchcock Medical Center, 2DHMC – Norris Cotton Cancer Center, 3Blood and Marrow Transplant Program, Norris Cotton Cancer Center, Dartmouth-Hitchcock Medical Center
Background: Allogeneic stem cell transplantation (Allo-SCT) is a potentially curative modality for many hematologic malignancies, due largely to the existence of an adoptive immunotherapeutic graft-versus-tumor effect. Recognition of this phenomenon has fostered implementation of novel conditioning regimens beyond traditional myeloablative regimens utilized as standard conditioning for many years. Accordingly, a novel conditioning regimen of Fludarabine/Busulfan in conjunction with Antithymocyte Globulin immunosuppression (Flu/Bu/ATG) was implemented at our center in 2012 for transplant of myeloid malignancies.
Methods: We retrospectively reviewed the post-transplant course and survival of 43 patients receiving an allo-SCT with Flu/Bu/ATG conditioning. Diagnoses included myeloproliferative neoplasm/myelofibrosis (n=8), acute leukemia (n=24), chronic leukemia (n=1), and myelodysplastic syndrome (n=10). Mean age at transplant was 57, with 16 matched related and 27 matched unrelated transplants performed.
Results: Mean post-transplant length of stay was 16 days, with mean ANC engraftment 13 days, and mean platelet engraftment 15 days. Acute GVHD incidence was 62.8% (≥ grade 3 in 25.6% of patients), and chronic GVHD incidence 25.6% (≥ grade 3 in 20.9% of patients). Survival at day 100 was 100% and at one year was 70%.
Conclusions: Allo-SCT utilizing the Flu/Bu/ATG regimen is an attractive alternative to traditional myeloablative conditioning for patients with myeloid malignancies. Its degree of immune suppression (to optimize engraftment), cytoreductive potential (to minimize relapse) and overall toxicity profile (to maximize tolerability) make it a promising combination for continued use in this challenging setting.
Relative and Absolute Platelet Count Drops as a Risk Factor for Mortality in Hospitalized Patients
Matthew E. Lebow1, Insu Koh, PhD1, Alicia Ellis, PhD2, Neil A. Zakai, MD, MSc1
1 University of Vermont, 2 Duke Clinical Research Institute
Introduction: Thrombocytopenia in hospitalized patients is common but poorly studied. There is a need to identify clinically meaningful predictors of mortality following hospitalized patients. We evaluated the incidence of hospital-acquired (HA) platelet count drops, determined the risk factors for HA platelet count drops in hospitalized patients and determined their association with mortality.
Methods: Data was abstracted from the electronic medical record database at the University of Vermont Medical Center, a 540- bed tertiary care hospital in Burlington, VT for admissions between 2009-16. The population was all adult patients (>18) admitted to a non-surgical service regardless of initial platelet count at admission. HA-platelet count drops were defined as relative drops and absolute platelet counts. A multivariable model predicting mortality was generated using a backward selection algorithm for risk factors present at admission with age and sex forced into the model, followed by addition of various changes in platelet counts as time-varying covariates.
Results: Over 7 years of follow up, there were 46,729 admissions and 9,936 deaths. In a development cohort of half the population, there were 23,364 admissions and 4,968 deaths.
Development of a Mortality Model: We assessed the following characteristics as potential risk factors for mortality: history of cancer, CHF (chronic), AIDS, history of venous thromboembolism (VTE), history of fracture (within 3 months of admission), history of respiratory dysfunction (requiring adjunct O2 therapy, including ventilatory assistance), diabetes mellitus, inflammatory disease, and COPD. We also looked at associations with mortality based on whether the patient at the time of admission had a current diagnosis of cancer, acute CHF exacerbation, or respiratory dysfunction. This also included accessing vital signs and lab values at the time of admission. After running our backward selection algorithm, the following risk factors were found to predict mortality; cancer (current and history of), AIDS, COPD, CHF exacerbation, respiratory dysfunction, low white blood count, low temperature, low systolic blood pressure. Length of admission, increasing age, female sex, and low BMI were also associated with increased mortality. The concordance index was calculated at 0.67.
Incidence of HA-Thrombocytopenia: Of 23,364 patients admitted, 1,952 (8.35%) developed a platelet count drop of greater than 100k, 12,397 (53.06%) had at least a 10% drop in their platelet count and 3,887(16.64%) had a relative drop of greater than 30% in their platelet count. While a lower baseline platelet count was found to be significantly associated with increased mortality, drops in platelet counts occurring during the hospitalization were protective both minimally adjusted and multivariable-adjusted models (See table).
Conclusion: We successfully developed a model for in-hospital mortality and demonstrated that factors occurring after admission are related to in-hospital mortality. The association of changes in platelet count with mortality were opposite those hypothesized, but consistent throughout the range of changes in platelet count. Further study will focus on different patient types (i.e. cancer patients, cardiac patients) and stratifying by baseline platelet count.
Disclosures: No relevant conflicts of interests to declare.
Table 1: Association of Hospital- Acquired Platelet Count Drops with Mortality
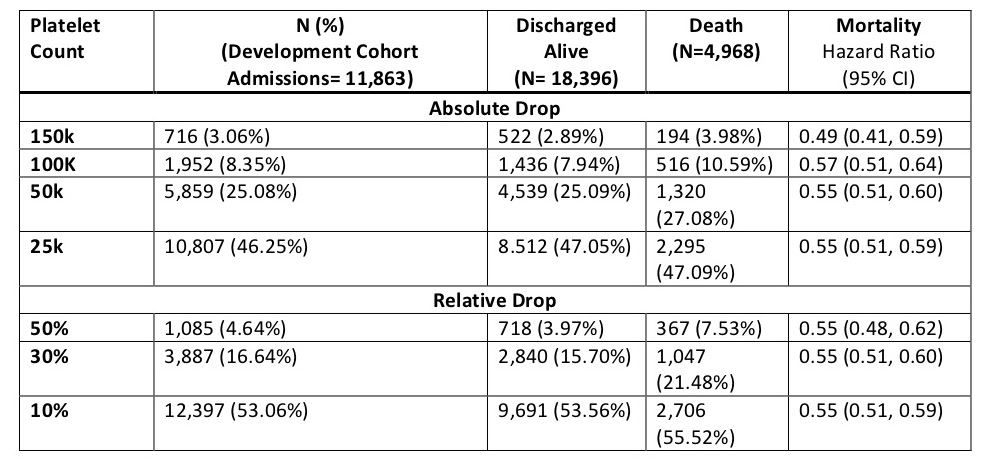
*Adjusted for Age, Sex, and Body Mass Index
Hematopoietic stem cell transplant for advanced myelofibrosis
D. R McKenna, C. H Lowrey, C.Ann Hayes, S. C Brighton, L. D Root, K. L Wilcox, E. Z Busnach, C.M Coughenour, J. M Hill, Jr, K. R Meehan
Blood and Marrow Transplant Program, Norris Cotton Cancer Center, Dartmouth-Hitchcock Medical Center
Background: Myelofibrosis is a rare cancer where the bone marrow is replaced by scar tissue. Median survival is less than 3 years. It can potentially be cured with an allogenic stem cell transplant.
Methods: We recently transplanted 11 patients with advanced myelofibrosis (primary myelofibrosis, n=6; myelofibrosis transformed to AML, n=2; MPN with myelofibrosis, n=2; MDS with myelofibrosis, n=1). The age at the time of transplant was 58 years (mean; range 42-69). All patients received relatively novel myeloablative conditioning with fludarabine, busulfan and anti-thymocyte globulin. There were 3 patients who received matched related donor cells and 8 patients who received matched unrelated donor cells. Pre-transplant spleen size ranged from “not palpable” to 32 finger-breadths below the costal margin.
Results: The length of stay was 26 days (mean, range 20-35) with engraftment of platelets on day 22 (mean; range 11-66) and ANC on day 14 (mean, range 11-23). Within 30 days of transplantation, the spleen size in 6 patients decreased an average of 79%. Post-transplant marrow fibrosis improved in 6 patients, and was stable in 3. Following transplant, all patients engrafted and all patients demonstrated complete donor chimerism by day 100. All patients with cytogenetic abnormalities in their original marrow normalized following transplant. The incidence of acute GVHD was 36% and 46% of patients developed chronic GVHD. Overall survival at day 100 was 100% and one year survival was 75%.
Conclusions: Although this is a small population, our results in this cohort with advanced myelofibrosis confirms the potentially curative effect of allogenic stem cell transplantation.
Prophylactic anticoagulation and bleeding risk in ambulatory cancer patients on cancer-directed therapy
Authors: A. Wilks, D.Douce, C. Holmes
Department of Medicine, Larner College of Medicine, University of Vermont
Background: Cancer is associated with an elevated risk for venous thromboembolism, however, prophylactic anticoagulation (AC) may lead to an increased risk of clinically significant bleeding events. We investigated whether ambulatory patients on cancer-directed therapies and prophylactic AC had an increased risk of clinically significant bleeds. We also investigated if the ATRIA bleeding risk score was predictive of an increased risk of bleeding events in these patients.
Methods: As part of a single-center prospective cohort study enrolling patients actively undergoing cancer-directed treatment and being prescribed prophylactic AC, bleeding events and patient demographics were gathered from the medical record. Bleeds and AC dosing were confirmed by physician review. Patients were enrolled from October 2015 through March 2018.
Results: The cohort consisted of 1,211 patients, and 82 bleeds were recorded: 44 major bleeds, 41 minor bleeds, and 6 with both a major and minor bleed. The odds ratio (OR) of developing a major bleed while on AC was 2.28 (95% CI 1.12, 4.38), while the OR associated with minor bleeding was 0.46 (95% CI 0.53, 2.79). The ATRIA score was not predictive of major or minor bleeding events, with an AUC of 0.438 (95% CI 0.35, 0.53).
Conclusions: Prophylactic AC in patients on cancer-directed therapy may result in an increased risk of major bleeding events. There was so significant increase in minor bleeding events. The ATRIA score was not accurate in predicting bleeding events in this cohort.


